Dynamics of Docosahexaenoic Acid Utilization by Mouse Peritoneal Macrophages
- PMID: 38002317
- PMCID: PMC10669016
- DOI: 10.3390/biom13111635
Dynamics of Docosahexaenoic Acid Utilization by Mouse Peritoneal Macrophages
Abstract
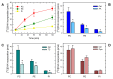
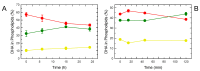
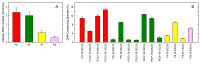
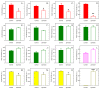
In this work, the incorporation of docosahexaenoic acid (DHA) in mouse resident peritoneal macrophages and its redistribution within the various phospholipid classes were investigated. Choline glycerophospholipids (PC) behaved as the major initial acceptors of DHA. Prolonged incubation with the fatty acid resulted in the transfer of DHA from PC to ethanolamine glycerophospholipids (PE), reflecting phospholipid remodeling. This process resulted in the cells containing similar amounts of DHA in PC and PE in the resting state. Mass spectrometry-based lipidomic analyses of phospholipid molecular species indicated a marked abundance of DHA in ether phospholipids. Stimulation of the macrophages with yeast-derived zymosan resulted in significant decreases in the levels of all DHA-containing PC and PI species; however, no PE or PS molecular species were found to decrease. In contrast, the levels of an unusual DHA-containing species, namely PI(20:4/22:6), which was barely present in resting cells, were found to markedly increase under zymosan stimulation. The levels of this phospholipid also significantly increased when the calcium-ionophore A23187 or platelet-activating factor were used instead of zymosan to stimulate the macrophages. The study of the route involved in the synthesis of PI(20:4/22:6) suggested that this species is produced through deacylation/reacylation reactions. These results define the increases in PI(20:4/22:6) as a novel lipid metabolic marker of mouse macrophage activation, and provide novel information to understand the regulation of phospholipid fatty acid turnover in activated macrophages.
Keywords: arachidonic acid; docosahexaenoic acid; inflammation; lipid signaling; membrane phospholipid; monocytes/macrophages.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

