A New Theory about Interfacial Proton Diffusion Revisited: The Commonly Accepted Laws of Electrostatics and Diffusion Prevail
- PMID: 38002323
- PMCID: PMC10669390
- DOI: 10.3390/biom13111641
A New Theory about Interfacial Proton Diffusion Revisited: The Commonly Accepted Laws of Electrostatics and Diffusion Prevail
Abstract
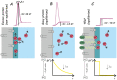
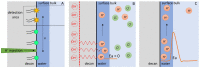
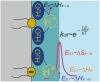
The high propensity of protons to stay at interfaces has attracted much attention over the decades. It enables long-range interfacial proton diffusion without relying on titratable residues or electrostatic attraction. As a result, various phenomena manifest themselves, ranging from spillover in material sciences to local proton circuits between proton pumps and ATP synthases in bioenergetics. In an attempt to replace all existing theoretical and experimental insight into the origin of protons' preference for interfaces, TELP, the "Transmembrane Electrostatically-Localized Protons" hypothesis, has been proposed. The TELP hypothesis envisions static H+ and OH- layers on opposite sides of interfaces that are up to 75 µm thick. Yet, the separation at which the electrostatic interaction between two elementary charges is comparable in magnitude to the thermal energy is more than two orders of magnitude smaller and, as a result, the H+ and OH- layers cannot mutually stabilize each other, rendering proton accumulation at the interface energetically unfavorable. We show that (i) the law of electroneutrality, (ii) Fick's law of diffusion, and (iii) Coulomb's law prevail. Using them does not hinder but helps to interpret previously published experimental results, and also helps us understand the high entropy release barrier enabling long-range proton diffusion along the membrane surface.
Keywords: PMF; TELP; interfacial water; proton diffusion; surface proton.
Conflict of interest statement
The authors declare no conflict of interest nor any competing financial interest.
Figures

Similar articles
-
Mitochondrial energetics with transmembrane electrostatically localized protons: do we have a thermotrophic feature?Sci Rep. 2021 Jul 16;11(1):14575. doi: 10.1038/s41598-021-93853-x. Sci Rep. 2021. PMID: 34272427 Free PMC article.
-
A critique of the capacitor-based "Transmembrane Electrostatically Localized Proton" hypothesis.J Bioenerg Biomembr. 2022 Apr;54(2):59-65. doi: 10.1007/s10863-022-09931-w. Epub 2022 Feb 21. J Bioenerg Biomembr. 2022. PMID: 35190945 Review.
-
Lee's transient protonic capacitor cannot explain the surface proton current observed in bacteriorhodopsin purple membranes.Biophys Chem. 2023 Oct;301:107096. doi: 10.1016/j.bpc.2023.107096. Epub 2023 Aug 15. Biophys Chem. 2023. PMID: 37604049
-
Lee's "Transmembrane Electrostatically-Localized Proton" model does NOT offer a better understanding of neuronal transmembrane potentials.J Neurophysiol. 2023 Jul 1;130(1):123-127. doi: 10.1152/jn.00173.2023. Epub 2023 Jun 14. J Neurophysiol. 2023. PMID: 37314087
-
Protons @ interfaces: implications for biological energy conversion.Biochim Biophys Acta. 2006 Aug;1757(8):913-30. doi: 10.1016/j.bbabio.2006.02.015. Epub 2006 Mar 24. Biochim Biophys Acta. 2006. PMID: 16624250 Review.
Cited by
-
Proton diffusion on the surface of mixed lipid membranes highlights the role of membrane composition.Biophys J. 2024 Dec 17;123(24):4200-4210. doi: 10.1016/j.bpj.2024.07.002. Epub 2024 Jul 2. Biophys J. 2024. PMID: 38961623
-
Calculation of proton transmembrane-electrostatic interaction force and elucidation of the water droplet experiment with a transient protonic front.RSC Adv. 2025 Jun 23;15(26):21108-21120. doi: 10.1039/d5ra03298a. eCollection 2025 Jun 16. RSC Adv. 2025. PMID: 40551833 Free PMC article.
-
Oxidative Phosphorylation Does Not Violate the Second Law of Thermodynamics.J Phys Chem B. 2024 Sep 5;128(35):8448-8458. doi: 10.1021/acs.jpcb.4c03047. Epub 2024 Aug 21. J Phys Chem B. 2024. PMID: 39167050 Free PMC article.
References
-
- Skulachev V. Current Topics in Bioenergetics. Volume 4. Elsevier; Amsterdam, The Netherlands: 1971. Energy transformations in the respiratory chain; pp. 127–190.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

