Plasma Androstenedione Concentration Can Discriminate Frail versus Non-Frail Men with Prostate Cancer under Androgen Deprivation Therapy
- PMID: 38002324
- PMCID: PMC10669122
- DOI: 10.3390/biom13111642
Plasma Androstenedione Concentration Can Discriminate Frail versus Non-Frail Men with Prostate Cancer under Androgen Deprivation Therapy
Abstract
Background: Androgen deprivation therapy (ADT) is a mainstay of prostate cancer in both adjuvant and palliative settings. Since androgens are crucial for functional status and psychological functions, we evaluated whether blood testosterone, androstenedione, or DHEA concentrations were associated with functional status and psychological alterations in patients with localised (PCa) or metastatic prostate cancer (mPCa) receiving ADT with analogues of luteinising hormone-releasing hormone (LHRH).
Methods: The five Fried criteria were considered to identify frailty syndrome. In addition, complementary evaluations were carried out to measure other variables of interest. Sleep quality was assessed using the Athens Insomnia Scale, cognitive functions were assessed using the Mini-Mental State Examination, and symptoms of depression were measured using the Yesavage Geriatric Depression Scale. Logistic regression analysis was performed to determine if the androgens level could be related to frailty syndrome, sleep impairment, depressive symptoms, and cognitive functions.
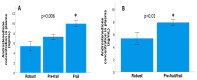
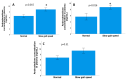
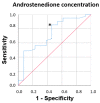
Results: The results of the multivariate analyses show that high concentrations of androstenedione were significantly associated with frailty syndrome in both groups (p = 0.018; odds ratio = 4.66, 95% confidence interval [1.30-16.6]). There were significant relationships between frailty syndrome and the systemic concentration of androstenedione (p = 0.01), but not the concentration of testosterone (p = 0.60) or DHEA (p = 0.42). In addition, the results of the non-parametric tests show significant results between a decreased gait speed in the two groups (metastatic and localised) and the concentration of androstenedione (p = 0.015). High androstenedione levels were associated with a slow walking speed in the mCaP group (p = 0.016), while high testosterone levels were associated with a better walking speed in the localised CaP group (p = 0.03). For the concentration of androstenedione in plasma, the area under the curve was 0.72, with a 95% CI of 0.55-0.88 with acceptable values, and with a cut-off point of 4.51 pg/mL, a sensitivity of 82.9%, and specificity of 53.8%. No relationships between the concentration of androgens in plasma and sleep quality, cognitive functions, or symptoms of depression suggest that the changes were specific to frailty syndrome.
Conclusions: Further research into the role of androstenedione should be evaluated in follow-up studies in order to recommend its use as a suitable biomarker of frailty syndrome in prostate cancer patients.
Keywords: DHEA; LHRH analogues; androstenedione; frailty syndrome; geriatric assessment; localised prostate cancer; metastatic prostate cancer; testosterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Comparison of Frailty Criteria, Cognitive Function, Depressive and Insomnia Symptoms in Men with Localized and Advanced Prostate Cancer under Androgen Deprivation Therapy.Healthcare (Basel). 2023 Apr 28;11(9):1266. doi: 10.3390/healthcare11091266. Healthcare (Basel). 2023. PMID: 37174808 Free PMC article.
-
Plasma IL-1β Concentration Associates with Sleep Quality and Cognitive Functions in Men with Prostate Cancer.Semin Oncol Nurs. 2025 Apr;41(2):151845. doi: 10.1016/j.soncn.2025.151845. Epub 2025 Feb 25. Semin Oncol Nurs. 2025. PMID: 40011135
-
Frailty syndrome is associated with changes in peripheral inflammatory markers in prostate cancer patients undergoing androgen deprivation therapy.Urol Oncol. 2019 Dec;37(12):976-987. doi: 10.1016/j.urolonc.2019.08.005. Epub 2019 Sep 12. Urol Oncol. 2019. PMID: 31521528 Clinical Trial.
-
Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer?: a conceptual review.Cancer. 2007 Dec 15;110(12):2604-13. doi: 10.1002/cncr.23084. Cancer. 2007. PMID: 17960609 Review.
-
[Cardiovascular risk patients under androgen deprivation therapy: Lower risk with GnRH antagonists compared to LHRH agonists?].Urologe A. 2016 Feb;55(2):218-25. doi: 10.1007/s00120-015-0013-1. Urologe A. 2016. PMID: 26637324 Review. German.
Cited by
-
Bibliometric analysis of research trends in the relationship between frailty and neoplasms over the past decade.Support Care Cancer. 2024 Jul 23;32(8):536. doi: 10.1007/s00520-024-08744-4. Support Care Cancer. 2024. PMID: 39042180 Free PMC article.
References
-
- Scher H.I., Halabi S., Tannock I., Morris M., Sternberg C.N., Carducci M.A., Eisenberger M.A., Higano C., Bubley G.J., Dreicer R., et al. Design and End Points of Clinical Trials for Patients with Progressive Prostate Cancer and Castrate Levels of Testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

