Non-Coding RNAs as Potential Targets for Diagnosis and Treatment of Oral Lichen Planus: A Narrative Review
- PMID: 38002328
- PMCID: PMC10669845
- DOI: 10.3390/biom13111646
Non-Coding RNAs as Potential Targets for Diagnosis and Treatment of Oral Lichen Planus: A Narrative Review
Abstract
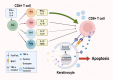
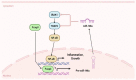
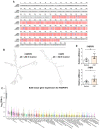
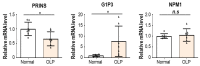
Oral lichen planus (OLP) is a chronic inflammatory disease that is characterized by the infiltration of T cells into the oral mucosa, causing the apoptosis of basal keratinocytes. OLP is a multifactorial disease of unknown etiology and is not solely caused by the malfunction of a single key gene but rather by various intracellular and extracellular factors. Non-coding RNAs play a critical role in immunological homeostasis and inflammatory response and are found in all cell types and bodily fluids, and their expression is closely regulated to preserve normal physiologies. The dysregulation of non-coding RNAs may be highly implicated in the onset and progression of diverse inflammatory disorders, including OLP. This narrative review summarizes the role of non-coding RNAs in molecular and cellular changes in the oral epithelium during OLP pathogenesis.
Keywords: T lymphocyte; circular RNA; inflammation; long non-coding RNA; microRNA; oral lichen planus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pathogenesis of oral lichen planus--a review.J Oral Pathol Med. 2010 Nov;39(10):729-34. doi: 10.1111/j.1600-0714.2010.00946.x. Epub 2010 Oct 4. J Oral Pathol Med. 2010. PMID: 20923445 Review.
-
Intraepithelial CD8+ lymphocytes as a predictive diagnostic biomarker for the remission of oral lichen planus.Hum Pathol. 2018 Apr;74:43-53. doi: 10.1016/j.humpath.2017.12.008. Epub 2017 Dec 28. Hum Pathol. 2018. PMID: 29288692
-
Immunohistochemical expression of cyclooxygenase-2 in oral lichen planus and normal oral mucosa.Indian J Pathol Microbiol. 2022 Jan-Mar;65(1):8-12. doi: 10.4103/IJPM.IJPM_1304_20. Indian J Pathol Microbiol. 2022. PMID: 35074958
-
Exploring the oral bacteria-oral lichen planus connection: mechanisms, clinical implications and future directions.Arch Microbiol. 2025 May 12;207(6):143. doi: 10.1007/s00203-025-04342-y. Arch Microbiol. 2025. PMID: 40353891 Review.
-
The possible roles of OPN-regulated CEACAM1 expression in promoting the survival of activated T cells and the apoptosis of oral keratinocytes in oral lichen planus patients.J Clin Immunol. 2011 Oct;31(5):827-39. doi: 10.1007/s10875-011-9552-4. Epub 2011 Jun 14. J Clin Immunol. 2011. PMID: 21671129
Cited by
-
Immunophenotypic and Gene Expression Analyses of the Inflammatory Microenvironment in High-Grade Oral Epithelial Dysplasia and Oral Lichen Planus.Head Neck Pathol. 2024 Mar 8;18(1):17. doi: 10.1007/s12105-024-01624-7. Head Neck Pathol. 2024. PMID: 38456941 Free PMC article.
-
Etiopathogenesis of Oral Lichen Planus: A Review.Head Neck Pathol. 2025 Jun 6;19(1):73. doi: 10.1007/s12105-025-01807-w. Head Neck Pathol. 2025. PMID: 40478312 Review.
-
Revolutionizing Dentistry: Preclinical Insights and Future Applications of mRNA Vaccines in Dentistry-A Narrative Review.Dent J (Basel). 2025 Feb 13;13(2):79. doi: 10.3390/dj13020079. Dent J (Basel). 2025. PMID: 39996953 Free PMC article. Review.
-
Long Noncoding RNA MIR4435-2HG is Involved in Osteogenic Differentiation and Inflammation in Human Dental Pulp Stem Cells.Int Dent J. 2025 Aug;75(4):100866. doi: 10.1016/j.identj.2025.100866. Epub 2025 Jun 25. Int Dent J. 2025. PMID: 40570674 Free PMC article.
-
Expression and clinical significance of CD155, FGL1, Galectin-9, and PD‑L1 in breast cancer with neoadjuvant chemotherapy.Med Oncol. 2025 Jul 25;42(9):375. doi: 10.1007/s12032-025-02914-y. Med Oncol. 2025. PMID: 40711645
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

