DPH1 Gene Mutations Identify a Candidate SAM Pocket in Radical Enzyme Dph1•Dph2 for Diphthamide Synthesis on EF2
- PMID: 38002337
- PMCID: PMC10669111
- DOI: 10.3390/biom13111655
DPH1 Gene Mutations Identify a Candidate SAM Pocket in Radical Enzyme Dph1•Dph2 for Diphthamide Synthesis on EF2
Abstract
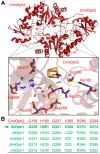
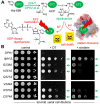
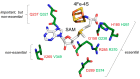
In eukaryotes, the Dph1•Dph2 dimer is a non-canonical radical SAM enzyme. Using iron-sulfur (FeS) clusters, it cleaves the cosubstrate S-adenosyl-methionine (SAM) to form a 3-amino-3-carboxy-propyl (ACP) radical for the synthesis of diphthamide. The latter decorates a histidine residue on elongation factor 2 (EF2) conserved from archaea to yeast and humans and is important for accurate mRNA translation and protein synthesis. Guided by evidence from archaeal orthologues, we searched for a putative SAM-binding pocket in Dph1•Dph2 from Saccharomyces cerevisiae. We predict an SAM-binding pocket near the FeS cluster domain that is conserved across eukaryotes in Dph1 but not Dph2. Site-directed DPH1 mutagenesis and functional characterization through assay diagnostics for the loss of diphthamide reveal that the SAM pocket is essential for synthesis of the décor on EF2 in vivo. Further evidence from structural modeling suggests particularly critical residues close to the methionine moiety of SAM. Presumably, they facilitate a geometry specific for SAM cleavage and ACP radical formation that distinguishes Dph1•Dph2 from classical radical SAM enzymes, which generate canonical 5'-deoxyadenosyl (dAdo) radicals.
Keywords: ADP ribosylation; Dph1•Dph2; EF2 diphthamide modification; SAM; Saccharomyces cerevisiae; diphtheria toxin; radical SAM enzymes.
Conflict of interest statement
K.M. and U.B. are employed by and members of Roche Pharma Research & Early Development (pRED) and are co-inventors on patent applications that cover assays to detect the presence or absence of diphthamide. Roche is interested in targeted therapies and diagnostics. All other authors declare no conflict of interest.
Figures


Similar articles
-
Functional Integrity of Radical SAM Enzyme Dph1•Dph2 Requires Non-Canonical Cofactor Motifs with Tandem Cysteines.Biomolecules. 2024 Apr 11;14(4):470. doi: 10.3390/biom14040470. Biomolecules. 2024. PMID: 38672486 Free PMC article.
-
The asymmetric function of Dph1-Dph2 heterodimer in diphthamide biosynthesis.J Biol Inorg Chem. 2019 Sep;24(6):777-782. doi: 10.1007/s00775-019-01702-0. Epub 2019 Aug 28. J Biol Inorg Chem. 2019. PMID: 31463593 Free PMC article.
-
Dph3 is an electron donor for Dph1-Dph2 in the first step of eukaryotic diphthamide biosynthesis.J Am Chem Soc. 2014 Feb 5;136(5):1754-7. doi: 10.1021/ja4118957. Epub 2014 Jan 22. J Am Chem Soc. 2014. PMID: 24422557 Free PMC article.
-
The diphthamide modification pathway from Saccharomyces cerevisiae--revisited.Mol Microbiol. 2014 Dec;94(6):1213-26. doi: 10.1111/mmi.12845. Epub 2014 Nov 17. Mol Microbiol. 2014. PMID: 25352115 Review.
-
Diphthamide - a conserved modification of eEF2 with clinical relevance.Trends Mol Med. 2024 Feb;30(2):164-177. doi: 10.1016/j.molmed.2023.11.008. Epub 2023 Dec 13. Trends Mol Med. 2024. PMID: 38097404 Review.
Cited by
-
Functional Integrity of Radical SAM Enzyme Dph1•Dph2 Requires Non-Canonical Cofactor Motifs with Tandem Cysteines.Biomolecules. 2024 Apr 11;14(4):470. doi: 10.3390/biom14040470. Biomolecules. 2024. PMID: 38672486 Free PMC article.
-
Bacterial exotoxins in medicine: potential value and perspectives.Int J Med Sci. 2025 Mar 31;22(9):2010-2019. doi: 10.7150/ijms.110104. eCollection 2025. Int J Med Sci. 2025. PMID: 40303490 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

