Furin Regulates the Alveolarization of Neonatal Lungs in a Mouse Model of Hyperoxic Lung Injury
- PMID: 38002338
- PMCID: PMC10669361
- DOI: 10.3390/biom13111656
Furin Regulates the Alveolarization of Neonatal Lungs in a Mouse Model of Hyperoxic Lung Injury
Abstract
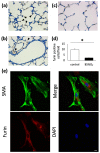
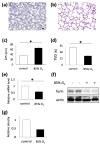
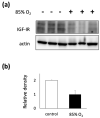
Despite advances in treatment options, such as corticosteroid administration and less invasive respiratory support, bronchopulmonary dysplasia (BPD) remains an important prognostic factor in preterm infants. We previously reported that furin regulates changes in lung smooth muscle cell phenotypes, suggesting that it plays a critical role in BPD pathogenesis. Therefore, in this study, we aimed to evaluate whether it regulates the alveolarization of immature lungs through activating alveolarization-driving proteins. We first examined furin expression levels, and its functions, using an established hyperoxia-induced BPD mouse model. Thereafter, we treated mice pups, as well as primary myofibroblast cell cultures, with furin inhibitors. Finally, we administered the hyperoxia-exposed mice pups with recombinant furin. Immunofluorescence revealed the co-expression of furin with alpha-smooth muscle actin. Hyperoxia exposure for 10 d decreased alveolar formation, as well as the expression of furin and its target, IGF-1R. Hexa-D-arginine administration also significantly inhibited alveolar formation. Another furin inhibitor, decanoyl-RVKR-chloromethylketone, accumulated pro-IGF-1R, and decreased IGF-1R phosphorylation in myofibroblast primary cultures. Finally, recombinant furin treatment significantly improved alveolarization in hyperoxia-exposed mice pups. Furin regulates alveolarization in immature lungs. Therefore, this study provides novel insights regarding the involvement of furin in BPD pathogenesis, and highlights a potential treatment target for ameliorating the impact of BPD.
Keywords: alveologenesis; bronchopulmonary dysplasia; proprotein convertase.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



Similar articles
-
Loss of microRNA-30a and sex-specific effects on the neonatal hyperoxic lung injury.Biol Sex Differ. 2023 Aug 8;14(1):50. doi: 10.1186/s13293-023-00535-6. Biol Sex Differ. 2023. PMID: 37553579 Free PMC article.
-
Treatment with Geranylgeranylacetone Induces Heat Shock Protein 70 and Attenuates Neonatal Hyperoxic Lung Injury in a Model of Bronchopulmonary Dysplasia.Lung. 2017 Aug;195(4):469-476. doi: 10.1007/s00408-017-0007-4. Epub 2017 Apr 26. Lung. 2017. PMID: 28447205 Free PMC article.
-
Recombinant CXCL17 Treatment Alleviates Hyperoxia-Induced Lung Apoptosis and Inflammation In Vivo and Vitro by Activating the AKT Pathway: A Possible Therapeutic Approach for Bronchopulmonary Dysplasia.Mol Biotechnol. 2024 Sep;66(9):2349-2361. doi: 10.1007/s12033-023-00866-0. Epub 2023 Sep 14. Mol Biotechnol. 2024. PMID: 37710083
-
The Role of Sphingolipid Signaling in Oxidative Lung Injury and Pathogenesis of Bronchopulmonary Dysplasia.Int J Mol Sci. 2022 Jan 23;23(3):1254. doi: 10.3390/ijms23031254. Int J Mol Sci. 2022. PMID: 35163176 Free PMC article. Review.
-
Hyperoxia-induced bronchopulmonary dysplasia: better models for better therapies.Dis Model Mech. 2021 Feb 23;14(2):dmm047753. doi: 10.1242/dmm.047753. Dis Model Mech. 2021. PMID: 33729989 Free PMC article. Review.
Cited by
-
Safety, Efficacy and Bio-Distribution Analysis of Exosomes Derived From Human Umbilical Cord Mesenchymal Stem Cells for Effective Treatment of Bronchopulmonary Dysplasia by Intranasal Administration in Mice Model.Int J Nanomedicine. 2025 Feb 27;20:2521-2553. doi: 10.2147/IJN.S501843. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40034220 Free PMC article.
References
-
- Stoll B.J., Hansen N.I., Bell E.F., Walsh M.C., Carlo W.A., Shankaran S., Laptook A.R., Sánchez P.J., Van Meurs K.P., Wyckoff M., et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. - DOI - PMC - PubMed
-
- Isayama T., Lee S.K., Yang J., Lee D., Daspal S., Dunn M., Shah P.S., Canadian Neonatal Network. Canadian Neonatal Follow-Up Network Investigators Revisiting the Definition of Bronchopulmonary Dysplasia: Effect of Changing Panoply of Respiratory Support for Preterm Neonates. JAMA Pediatr. 2017;171:271–279. doi: 10.1001/jamapediatrics.2016.4141. - DOI - PubMed
-
- Schlapbach L.J., Adams M., Proietti E., Aebischer M., Grunt S., Borradori-Tolsa C., Bickle-Graz M., Bucher H.U., Latal B., Natalucci G. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr. 2012;12:198. doi: 10.1186/1471-2431-12-198. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

