Visible Light-Induced Templated Metathesis of Peptide-Nucleic Acid Conjugates with a Diselenide Bridge
- PMID: 38002358
- PMCID: PMC10669671
- DOI: 10.3390/biom13111676
Visible Light-Induced Templated Metathesis of Peptide-Nucleic Acid Conjugates with a Diselenide Bridge
Abstract
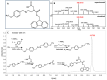
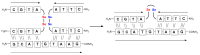
The use of template molecules as chemical scaffolds that significantly influence the course of the reaction has recently been intensively studied. Peptide nucleic acids (PNA) are molecules that mimic natural nucleic acids. They are a promising matrix in such reactions because they possess high affinity and specificity in their interactions. The manner of PNA interaction is predictable based on sequence complementarity. Recently, we report the visible light-induced metathesis reaction in peptides containing a diselenide bond. Herein, we present an efficient and straightforward method of the visible light-driven diselenide-based metathesis of peptide-nucleic acid conjugates. Compared to a similar photochemical transformation in peptides, a significant increase in the metathesis efficiency was obtained due to the template effect.
Keywords: metathesis; peptide nucleic acid; selenium; templated synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
RNA-templated chemical synthesis of proapoptotic L- and d-peptides.Bioorg Med Chem. 2022 Jul 15;66:116786. doi: 10.1016/j.bmc.2022.116786. Epub 2022 May 14. Bioorg Med Chem. 2022. PMID: 35594647
-
Native Chemical Ligation Directed by Photocleavable Peptide Nucleic Acid (PNA) Templates.Chembiochem. 2017 Dec 5;18(23):2328-2332. doi: 10.1002/cbic.201700487. Epub 2017 Nov 2. Chembiochem. 2017. PMID: 28987009
-
Towards a templated reaction that translates RNA in cells into a proaptotic peptide-PNA conjugate.J Pept Sci. 2023 Jul;29(7):e3477. doi: 10.1002/psc.3477. Epub 2023 Jan 20. J Pept Sci. 2023. PMID: 36606596
-
Peptide nucleic acid conjugates and their antimicrobial applications-a mini-review.Eur Biophys J. 2023 Oct;52(6-7):533-544. doi: 10.1007/s00249-023-01673-w. Epub 2023 Aug 23. Eur Biophys J. 2023. PMID: 37610696 Free PMC article. Review.
-
Applications of peptide nucleic acids (PNAs) and locked nucleic acids (LNAs) in biosensor development.Anal Bioanal Chem. 2012 Apr;402(10):3071-89. doi: 10.1007/s00216-012-5742-z. Anal Bioanal Chem. 2012. PMID: 22297860 Review.
Cited by
-
Shining a Light on Peptide and Protein Synthesis: Light-Emitting-Diode-Driven Desulfurization of Cysteine to Alanine with Rose Bengal.Org Lett. 2025 Feb 7;27(5):1159-1163. doi: 10.1021/acs.orglett.4c04671. Epub 2025 Jan 23. Org Lett. 2025. PMID: 39848618 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

