A Glutaraldehyde-Free Crosslinking Method for the Treatment of Collagen-Based Biomaterials for Clinical Application
- PMID: 38002371
- PMCID: PMC10669889
- DOI: 10.3390/bioengineering10111247
A Glutaraldehyde-Free Crosslinking Method for the Treatment of Collagen-Based Biomaterials for Clinical Application
Abstract
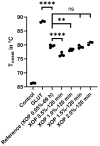
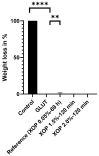
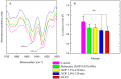
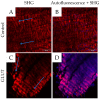
Biological bioprostheses such as grafts, patches, and heart valves are often derived from biological tissue like the pericardium. These bioprostheses can be of xenogenic, allogeneic, or autologous origin. Irrespective of their origin, all types are pre-treated via crosslinking to render the tissue non-antigenic and mechanically strong or to minimize degradation. The most widely used crosslinking agent is glutaraldehyde. However, glutaraldehyde-treated tissue is prone to calcification, inflammatory degradation, and mechanical injury, and it is incapable of matrix regeneration, leading to structural degeneration over time. In this work, we are investigating an alternative crosslinking method for an intraoperative application. The treated tissue's crosslinking degree was evaluated by differential scanning calorimetry. To confirm the findings, a collagenase assay was conducted. Uniaxial tensile testing was used to assess the tissue's mechanical properties. To support the findings, the treated tissue was visualized using two-photon microscopy. Additionally, fourier transform infrared spectroscopy was performed to study the overall protein secondary structure. Finally, a crosslinking procedure was identified for intraoperative processing. The samples showed a significant increase in thermal and enzymatic stability after treatment compared to the control, with a difference of up to 22.2 °C and 100%, respectively. Also, the tissue showed similar biomechanics to glutaraldehyde-treated tissue, showing greater extensibility, a higher failure strain, and a lower ultimate tensile strength than the control. The significant difference in the structure band ratio after treatment is proof of the introduction of additional crosslinks compared to the untreated control with regard to differences in the amide-I region. The microscopic images support these findings, showing an alteration of the fiber orientation after treatment. For collagen-based biomaterials, such as pericardial tissue, the novel phenolic crosslinking agent proved to be an equivalent alternative to glutaraldehyde regarding tissue characteristics. Although long-term studies must be performed to investigate superiority in terms of longevity and calcification, our novel crosslinking agent can be applied in concentrations of 1.5% or 2.0% for the treatment of biomaterials.
Keywords: biomaterials; biomedical devices; collagen; crosslinking; glutaraldehyde-free; implantology; pericardium; regenerative medicine; tissue application.
Conflict of interest statement
The first author, as well as some secondary authors, are employed by GrOwnValve GmbH.
Figures



Similar articles
-
Biaxial mechanical/structural effects of equibiaxial strain during crosslinking of bovine pericardial xenograft materials.Biomaterials. 1999 Jan;20(2):137-53. doi: 10.1016/s0142-9612(98)00142-2. Biomaterials. 1999. PMID: 10022783
-
Infrared Spectroscopic Verification of a α-Helical Collagen Structure in Glutaraldehyde-Free Crosslinked Bovine Pericardium for Cardiac Implants.Life (Basel). 2022 Dec 6;12(12):2035. doi: 10.3390/life12122035. Life (Basel). 2022. PMID: 36556400 Free PMC article.
-
Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent.J Biomed Mater Res. 1999 Nov;47(2):116-26. doi: 10.1002/(sici)1097-4636(199911)47:2<116::aid-jbm2>3.0.co;2-j. J Biomed Mater Res. 1999. PMID: 10449623
-
Lysine-enhanced glutaraldehyde crosslinking of collagenous biomaterials.J Biomed Mater Res. 1991 Dec;25(12):1495-505. doi: 10.1002/jbm.820251207. J Biomed Mater Res. 1991. PMID: 1794997
-
Solvent environment modulates effects of glutaraldehyde crosslinking on tissue-derived biomaterials.J Biomed Mater Res. 1996 Aug;31(4):533-43. doi: 10.1002/(SICI)1097-4636(199608)31:4<533::AID-JBM14>3.0.CO;2-H. J Biomed Mater Res. 1996. PMID: 8836851
Cited by
-
Development of Biomimetic Substrates for Limbal Epithelial Stem Cells Using Collagen-Based Films, Hyaluronic Acid, Immortalized Cells, and Macromolecular Crowding.Life (Basel). 2024 Nov 26;14(12):1552. doi: 10.3390/life14121552. Life (Basel). 2024. PMID: 39768260 Free PMC article.
-
Cross-Linking Agents in Three-Component Materials Dedicated to Biomedical Applications: A Review.Polymers (Basel). 2024 Sep 23;16(18):2679. doi: 10.3390/polym16182679. Polymers (Basel). 2024. PMID: 39339142 Free PMC article. Review.
-
Ultramodern natural and synthetic polymer hydrogel scaffolds for articular cartilage repair and regeneration.Biomed Eng Online. 2025 Feb 7;24(1):13. doi: 10.1186/s12938-025-01342-3. Biomed Eng Online. 2025. PMID: 39920742 Free PMC article. Review.
References
-
- Straka F., Schornik D., Masin J., Filova E., Mirejovsky T., Burdikova Z., Svindrych Z., Chlup H., Horny L., Daniel M., et al. A human pericardium biopolymeric scaffold for autologous heart valve tissue engineering: Cellular and extracellular matrix structure and biomechanical properties in comparison with a normal aortic heart valve. J. Biomater. Sci. Polym. Ed. 2018;29:599–634. doi: 10.1080/09205063.2018.1429732. - DOI - PubMed
-
- Remi E., Khelil N., Di I., Roques C., Ba M., Medjahed-Hamidi F., Chaubet F., Letourneur D., Lansac E., Meddahi-Pelle A. Biomaterials Science and Engineering. IntechOpen; London, UK: 2011. Pericardial Processing: Challenges, Outcomes and Future Prospects. - DOI
-
- Balguid A., Rubbens M.P., Mol A., Bank R.A., Bogers A.J., van Kats J.P., de Mol B.A., Baaijens F.P.T., Bouten C.V.C. The Role of Collagen Cross-Links in Biomechanical Behavior of Human Aortic Heart Valve Leaflets—Relevance for Tissue Engineering. Tissue Eng. 2007;13:1501–1511. doi: 10.1089/ten.2006.0279. - DOI - PubMed
-
- Taylor P.M., Allen S.P., Yacoub M.H. Phenotypic and Functional Characterization of Interstitial Cells from Human Heart Valves, Pericardium and Skin. J. Heart Valve Dis. 2000;9:150–158. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

