Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix
- PMID: 38002395
- PMCID: PMC10669840
- DOI: 10.3390/bioengineering10111271
Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix
Abstract
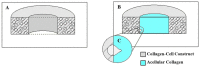
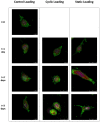
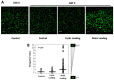
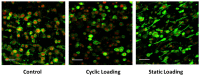
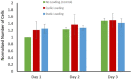
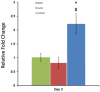
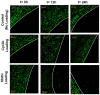
This study aimed to understand extracellular mechanical stimuli's effect on prostate cancer cells' metastatic progression within a three-dimensional (3D) bone-like microenvironment. In this study, a mechanical loading platform, EQUicycler, has been employed to create physiologically relevant static and cyclic mechanical stimuli to a prostate cancer cell (PC-3)-embedded 3D tissue matrix. Three mechanical stimuli conditions were applied: control (no loading), cyclic (1% strain at 1 Hz), and static mechanical stimuli (1% strain). The changes in prostate cancer cells' cytoskeletal reorganization, polarity (elongation index), proliferation, expression level of N-Cadherin (metastasis-associated gene), and migratory potential within the 3D collagen structures were assessed upon mechanical stimuli. The results have shown that static mechanical stimuli increased the metastasis progression factors, including cell elongation (p < 0.001), cellular F-actin accumulation (p < 0.001), actin polymerization (p < 0.001), N-Cadherin gene expression, and invasion capacity of PC-3 cells within a bone-like microenvironment compared to its cyclic and control loading counterparts. This study established a novel system for studying metastatic cancer cells within bone and enables the creation of biomimetic in vitro models for cancer research and mechanobiology.
Keywords: EQUicycler; actin; bone; cancer; cyclic; cytoskeleton; elongation; extracellular; invasion; loading; mechanical; metastasis; prostate; static; stimuli.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Caley M.P., King H., Shah N., Wang K., Rodriguez-Teja M., Gronau J.H., Waxman J., Sturge J. Tumor-associated Endo180 requires stromal-derived LOX to promote metastatic prostate cancer cell migration on human ECM surfaces. Clin. Exp. Metastasis. 2016;33:151–165. doi: 10.1007/s10585-015-9765-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

