Plant Cellulose as a Substrate for 3D Neural Stem Cell Culture
- PMID: 38002433
- PMCID: PMC10669287
- DOI: 10.3390/bioengineering10111309
Plant Cellulose as a Substrate for 3D Neural Stem Cell Culture
Abstract
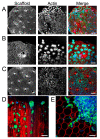
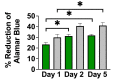
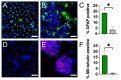
Neural stem cell (NSC)-based therapies are at the forefront of regenerative medicine strategies for various neural defects and injuries such as stroke, traumatic brain injury, and spinal cord injury. For several clinical applications, NSC therapies require biocompatible scaffolds to support cell survival and to direct differentiation. Here, we investigate decellularized plant tissue as a novel scaffold for three-dimensional (3D), in vitro culture of NSCs. Plant cellulose scaffolds were shown to support the attachment and proliferation of adult rat hippocampal neural stem cells (NSCs). Further, NSCs differentiated on the cellulose scaffold had significant increases in their expression of neuron-specific beta-III tubulin and glial fibrillary acidic protein compared to 2D culture on a polystyrene plate, indicating that the scaffold may enhance the differentiation of NSCs towards astrocytic and neuronal lineages. Our findings suggest that plant-derived cellulose scaffolds have the potential to be used in neural tissue engineering and can be harnessed to direct the differentiation of NSCs.
Keywords: 3D cell culture scaffold; NSC; biomaterial.
Conflict of interest statement
L.J.C., K.L.A.W. and A.E.P. are inventors on a patent application which describes PLO coating of plant-based biomaterials.
Figures

Similar articles
-
Release of O-GlcNAc transferase inhibitor promotes neuronal differentiation of neural stem cells in 3D bioprinted supramolecular hydrogel scaffold for spinal cord injury repair.Acta Biomater. 2022 Oct 1;151:148-162. doi: 10.1016/j.actbio.2022.08.031. Epub 2022 Aug 21. Acta Biomater. 2022. PMID: 36002129
-
Inhibited astrocytic differentiation in neural stem cell-laden 3D bioprinted conductive composite hydrogel scaffolds for repair of spinal cord injury.Biomater Adv. 2023 May;148:213385. doi: 10.1016/j.bioadv.2023.213385. Epub 2023 Mar 14. Biomater Adv. 2023. PMID: 36934714
-
3D culture of neural stem cells within conductive PEDOT layer-assembled chitosan/gelatin scaffolds for neural tissue engineering.Mater Sci Eng C Mater Biol Appl. 2018 Dec 1;93:890-901. doi: 10.1016/j.msec.2018.08.054. Epub 2018 Aug 29. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 30274126
-
Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair.Cells. 2022 Mar 1;11(5):846. doi: 10.3390/cells11050846. Cells. 2022. PMID: 35269466 Free PMC article. Review.
-
Scaffold-free 3D culture systems for stem cell-based tissue regeneration.APL Bioeng. 2024 Oct 1;8(4):041501. doi: 10.1063/5.0225807. eCollection 2024 Dec. APL Bioeng. 2024. PMID: 39364211 Free PMC article. Review.
Cited by
-
Investigation of Persistent Photoconductivity of Gallium Nitride Semiconductor and Differentiation of Primary Neural Stem Cells.Molecules. 2024 Sep 19;29(18):4439. doi: 10.3390/molecules29184439. Molecules. 2024. PMID: 39339434 Free PMC article.
References
-
- Zhao X.-M., He X.-Y., Liu J., Xu Y., Xu F.-F., Tan Y.-X., Zhang Z.-B., Wang T.-H. Neural Stem Cell Transplantation Improves Locomotor Function in Spinal Cord Transection Rats Associated with Nerve Regeneration and IGF-1 R Expression. Cell Transplant. 2019;28:1197–1211. doi: 10.1177/0963689719860128. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

