Pharmaceutical Interventions for Inpatients with Liver Cirrhosis and Liver Transplantation: A Systematic Review of Experimental Studies
- PMID: 38002644
- PMCID: PMC10671912
- DOI: 10.3390/jcm12227030
Pharmaceutical Interventions for Inpatients with Liver Cirrhosis and Liver Transplantation: A Systematic Review of Experimental Studies
Abstract
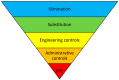
Liver cirrhosis, which is considered one of the leading causes of death in the world, can lead to severe complications, and is often followed by a liver transplantation. These patients take an average of nine medications daily. If not managed adequately, it can be accompanied by serious drug-related problems. To reduce this risk, a clinical pharmacist may be included as part of the healthcare team to optimize medication therapy in this population. This study aimed to systematically identify the pharmaceutical interventions which reduced drug-related problems and improved medication therapy for adult hospitalized liver cirrhotic and liver transplant patients when compared to standard care. Three databases (PubMed, Embase, and CENTRAL) were systematically searched from the inception of each database to 25 October 2023, and interventional studies in the English language were included. The risk of bias was assessed according to RoB-I for the UBA study and RoB2 for the identified RCT. The detected interventions to reduce drug-related problems in liver cirrhotic and liver transplant patients were extracted and classified according to a "Hierarchy of Controls" model. Two studies from Germany and the USA met our inclusion criteria, respectively. In these studies, we identified two interventions that included education, expert consultation, and the monitoring of the immunosuppressive medications serum level. The main objective of the two included studies was improving patients' compliance through adherence. These pharmaceutical interventions identified were classified as administrative controls, which is one of the lowest levels in the "Hierarchy of Controls" with which to address a potential risk. Pharmaceutical interventions to optimize medication therapy were found to be rare in the examined population, and were limited to "administrative controls". These interventions were limited to transplant patients' education and the monitoring of the immunosuppressive medication serum levels. No interventional studies were found to have investigated pharmaceutical interventions in patients with liver cirrhosis. Especially regarding this patient group, future studies to reduce DRPs using pharmaceutical interventions are needed. This study received no external funding and its PROSPERO registration number is CRD42022309122.
Keywords: drug-related problems; experimental study; liver cirrhosis; liver transplantation; pharmaceutical care.
Conflict of interest statement
The authors declare there are no conflict of interest.
Figures



Similar articles
-
Risk Factors Associated with the Requirement for Pharmaceutical Intervention in the Hospital Setting: A Systematic Review of the Literature.Drugs Real World Outcomes. 2016 Sep;3(3):241-263. doi: 10.1007/s40801-016-0083-4. Drugs Real World Outcomes. 2016. PMID: 27747829 Free PMC article. Review.
-
Recovery schools for improving behavioral and academic outcomes among students in recovery from substance use disorders: a systematic review.Campbell Syst Rev. 2018 Oct 4;14(1):1-86. doi: 10.4073/csr.2018.9. eCollection 2018. Campbell Syst Rev. 2018. PMID: 37131375 Free PMC article.
-
Intensified pharmaceutical care is improving immunosuppressive medication adherence in kidney transplant recipients during the first post-transplant year: a quasi-experimental study.Nephrol Dial Transplant. 2014 Aug;29(8):1597-607. doi: 10.1093/ndt/gfu207. Epub 2014 Jun 9. Nephrol Dial Transplant. 2014. PMID: 24914089 Clinical Trial.
-
Systematic Pharmaceutical Educational Approach to Enhance Drug Adherence in Liver Transplant Recipients.Transplant Proc. 2016 May;48(4):1202-7. doi: 10.1016/j.transproceed.2015.12.100. Transplant Proc. 2016. PMID: 27320587
-
Interventions to Reduce Medication Dispensing, Administration, and Monitoring Errors in Pediatric Professional Healthcare Settings: A Systematic Review.Front Pediatr. 2021 May 26;9:633064. doi: 10.3389/fped.2021.633064. eCollection 2021. Front Pediatr. 2021. PMID: 34123962 Free PMC article.
References
-
- Tsai T.Y., Hung T.H., Livneh H., Lin I.H., Lu M.C., Yeh C.C. Chinese herbal medicine therapy and the risk of mortality for chronic hepatitis B patients with concurrent liver cirrhosis: A nationwide population-based cohort study. Oncotarget. 2018;9:18214–18223. doi: 10.18632/oncotarget.24383. - DOI - PMC - PubMed
-
- Martin Blachier H.L., Peck-Radosavljevic M., Valla D.-C., Roudot-Thoraval F. The Burden of Liver Disease in Europe A Review of Available Epidemiological Data. EASL; Geneva, Switzerland: 2013. - PubMed
-
- (WHO), W.H.O Alcohol in the European Union: Consumption, Harm and Policy Approaches. [(accessed on 27 September 2023)]. Available online: https://www.euro.who.int/__data/assets/pdf_file/0003/160680/e96457.pdf.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

