Pharmacoepidemiology: An Overview
- PMID: 38002647
- PMCID: PMC10672708
- DOI: 10.3390/jcm12227033
Pharmacoepidemiology: An Overview
Abstract
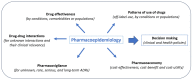
The aims of this review are to provide a comprehensive overview of the definition and scope of pharmacoepidemiology, to summarize the study designs and methodologies used in the field, to discuss the future trends in the field and new methodologies to address bias and confounding, and finally to give some recommendations to clinicians interested in pharmacoepidemiologic research. Because drug efficacy and safety from randomized clinical trials do not reflect the real-world situation, pharmacoepidemiological studies on drug safety monitoring and drug effectiveness in large numbers of people are needed by healthcare professionals and regulatory institutions. We aim to highlight the importance of pharmacoepidemiologic research in informing evidence-based medicine and public health policy. The development of new designs and methodologies for the generation of valid evidence, as well as new initiatives to provide guidance and recommendations on how to incorporate real-world evidence into the drug development process, are reported on. In addition, we have touched on the implication of artificial intelligence in the management of real-world data. This overview aims to summarize all important aspects to consider when conducting or interpreting a pharmacoepidemiologic study.
Keywords: adverse drug reaction; drug safety; effectiveness; patient safety; pharmacoepidemiology; pharmacovigilance; regulatory decision.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources