Short-Term Evaluation of Bone-ACL-Bone Complex Allograft in ACL Reconstruction in a Rabbit Model
- PMID: 38002670
- PMCID: PMC10671951
- DOI: 10.3390/jcm12227057
Short-Term Evaluation of Bone-ACL-Bone Complex Allograft in ACL Reconstruction in a Rabbit Model
Abstract
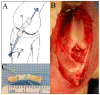
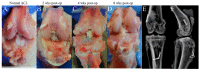
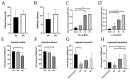
The study is to evaluate incorporation of a bone-anterior cruciate ligament-bone (B-ACL-B) allograft in anterior cruciate ligament (ACL) reconstruction in a rabbit model. A total of 61 New Zealand white rabbits were used, with 23 donor rabbits for harvesting B-ACL-B allografts and 38 recipient rabbits undergoing unilateral ACL reconstruction with B-ACL-B allograft. Animals were euthanized for biomechanical testing, micro-computed tomography examination, histological analysis, multi-photon microscopy and transmission electron microscopy testing at 2, 4 and 8 weeks after surgery. Gross inspection and radiographs confirmed the intact ACL allograft in the proper anatomic position. Progressive healing occurred between the bone block and the bone tunnel as demonstrated by a gradual increase in average bone volume fraction and total mineral density at 4 and 8 weeks. Histological analysis showed new bone formation at the bone block-tunnel interface, with maintenance of the native ACL enthesis. Ultrastructural analysis demonstrated the maintenance of overall collagen matrix alignment, while there was repopulation with smaller diameter collagen fibrils. There was no significant difference between 4 and 8 weeks in mean failure force (p = 0.39) or stiffness (p = 0.15) for the B-ACL-B allografts. This study demonstrates the restoration of the normal anatomy of the ACL and progressive graft incorporation and remodeling using a B-ACL-B allograft for ACL reconstruction in the rabbit knee.
Keywords: allograft; animal model; anterior cruciate ligament reconstruction; bone–ACL–bone; healing.
Conflict of interest statement
This study received research support from JRF Ortho. Dr. Rodeo has potential conflicts of interest due to stock options in Ortho RTI and consulting in Advance Medical/Teladoc. Other authors certify that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose as a conflict of interest in connection with the submitted article related to the author or any immediate family members. The work has been presented as a poster in the ORS and AOSSM 2022 annual meeting.
Figures





Similar articles
-
Bone-ACL-bone allograft for anterior cruciate ligament reconstruction: Short-term evaluation in a rabbit model with microcomputed tomography.J Orthop Res. 2023 Aug;41(8):1697-1708. doi: 10.1002/jor.25520. Epub 2023 Feb 5. J Orthop Res. 2023. PMID: 36691866
-
Decellularized Versus Fresh-Frozen Allografts in Anterior Cruciate Ligament Reconstruction: An In Vitro Study in a Rabbit Model.Am J Sports Med. 2015 Aug;43(8):1924-34. doi: 10.1177/0363546515585314. Epub 2015 Jun 2. Am J Sports Med. 2015. PMID: 26037623
-
The Effect of Graft Pretensioning on Bone Tunnel Diameter and Bone Formation After Anterior Cruciate Ligament Reconstruction in a Rat Model: Evaluation With Micro-Computed Tomography.Am J Sports Med. 2017 May;45(6):1349-1358. doi: 10.1177/0363546516686967. Epub 2017 Feb 1. Am J Sports Med. 2017. PMID: 28298055
-
Two-Stage Revision Anterior Cruciate Ligament Reconstruction: A Systematic Review of Bone Graft Options for Tunnel Augmentation.Am J Sports Med. 2020 Mar;48(3):767-777. doi: 10.1177/0363546519841583. Epub 2019 May 22. Am J Sports Med. 2020. PMID: 31116949
-
Anterior cruciate ligament reconstruction using semitendinosus and gracilis tendons, bone patellar tendon, or quadriceps tendon-graft with press-fit fixation without hardware. A new and innovative procedure.Orthop Clin North Am. 2003 Jan;34(1):49-64. doi: 10.1016/s0030-5898(02)00070-6. Orthop Clin North Am. 2003. PMID: 12735201 Review.
Cited by
-
Early clinical outcomes of all-inside arthroscopic anterior cruciate ligament reconstruction with autograft tendon augmentation using the LARS internal brace ligament.Front Bioeng Biotechnol. 2025 Apr 15;13:1556106. doi: 10.3389/fbioe.2025.1556106. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40304007 Free PMC article.
References
-
- Rodeo S.A., Kawamura S., Ma C.B., Deng X., Sussman P.S., Patrick S., Hays P., Ying L. The effect of osteoclastic activity on tendon-to-bone healing: An experimental study in rabbits. J. Bone Jt. Surg. Am. 2007;89:2250–2259. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

