Gut Microbiota to Microglia: Microbiome Influences Neurodevelopment in the CNS
- PMID: 38002858
- PMCID: PMC10670365
- DOI: 10.3390/children10111767
Gut Microbiota to Microglia: Microbiome Influences Neurodevelopment in the CNS
Abstract
The brain is traditionally viewed as an immunologically privileged site; however, there are known to be multiple resident immune cells that influence the CNS environment and are reactive to extra-CNS signaling. Microglia are an important component of this system, which influences early neurodevelopment in addition to modulating inflammation and regenerative responses to injury and infection. Microglia are influenced by gut microbiome-derived metabolites, both as part of their normal function and potentially in pathological patterns that may induce neurodevelopmental disabilities or behavioral changes. This review aims to summarize the mounting evidence indicating that, not only is the Gut-Brain axis mediated by metabolites and microglia throughout an organism's lifetime, but it is also influenced prenatally by maternal microbiome and diet, which holds implications for both early neuropathology and neurodevelopment.
Keywords: Gut–Brain axis; gut microbiome; metabolite; microglia; neurodevelopment.
Conflict of interest statement
Ajay K. Jain is a consultant for Mirum Pharmaceuticals. The remaining authors declare no conflict of interest.
Figures
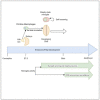
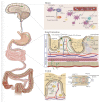
Similar articles
-
Microglia and Microbiome-Gut-Brain Axis.Adv Neurobiol. 2024;37:303-331. doi: 10.1007/978-3-031-55529-9_17. Adv Neurobiol. 2024. PMID: 39207699 Review.
-
The Gut-Microglia Connection: Implications for Central Nervous System Diseases.Front Immunol. 2018 Oct 5;9:2325. doi: 10.3389/fimmu.2018.02325. eCollection 2018. Front Immunol. 2018. PMID: 30344525 Free PMC article. Review.
-
Microglia: Immune Regulators of Neurodevelopment.Front Immunol. 2018 Nov 7;9:2576. doi: 10.3389/fimmu.2018.02576. eCollection 2018. Front Immunol. 2018. PMID: 30464763 Free PMC article. Review.
-
Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis.Biomed Pharmacother. 2023 Apr;160:114308. doi: 10.1016/j.biopha.2023.114308. Epub 2023 Jan 28. Biomed Pharmacother. 2023. PMID: 36709599
-
Regulation of microglial physiology by the microbiota.Gut Microbes. 2022 Jan-Dec;14(1):2125739. doi: 10.1080/19490976.2022.2125739. Gut Microbes. 2022. PMID: 36151874 Free PMC article. Review.
Cited by
-
Intraduodenal fecal microbiota transplantation ameliorates gut atrophy and cholestasis in a novel parenteral nutrition piglet model.Am J Physiol Gastrointest Liver Physiol. 2024 Nov 1;327(5):G640-G654. doi: 10.1152/ajpgi.00012.2024. Epub 2024 Aug 20. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 39163019
-
Unravelling the Gut-Microbiome-Brain Axis: Implications for Infant Neurodevelopment and Future Therapeutics.Curr Microbiol. 2025 Jul 16;82(9):390. doi: 10.1007/s00284-025-04370-3. Curr Microbiol. 2025. PMID: 40670809 Review.
-
Gut microbiota: A new window for the prevention and treatment of neuropsychiatric disease.J Cent Nerv Syst Dis. 2025 Feb 21;17:11795735251322450. doi: 10.1177/11795735251322450. eCollection 2025. J Cent Nerv Syst Dis. 2025. PMID: 39989718 Free PMC article. Review.
-
The gut microbiota regulates diabetic retinopathy in adult rats.Front Microbiol. 2025 Jan 29;16:1479792. doi: 10.3389/fmicb.2025.1479792. eCollection 2025. Front Microbiol. 2025. PMID: 39949626 Free PMC article.
-
Nanoplastics and Neurodegeneration in ALS.Brain Sci. 2024 May 7;14(5):471. doi: 10.3390/brainsci14050471. Brain Sci. 2024. PMID: 38790450 Free PMC article. Review.
References
-
- Luczynski P., Whelan S.O., O’Sullivan C., Clarke G., Shanahan F., Dinan T.G., Cryan J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016;44:2654–2666. doi: 10.1111/ejn.13291. - DOI - PMC - PubMed
-
- Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. Corrected in Sci. Transl. Med. 2014, 6, 266er7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

