Analysis of Adverse Drug Reactions in Pediatric Patients with Epilepsy: An Intensive Pharmacovigilance Study
- PMID: 38002866
- PMCID: PMC10670375
- DOI: 10.3390/children10111775
Analysis of Adverse Drug Reactions in Pediatric Patients with Epilepsy: An Intensive Pharmacovigilance Study
Abstract
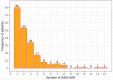
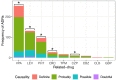
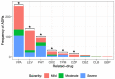
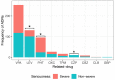
Epilepsy is a chronic neurological disease characterized by the presence of spontaneous seizures, with a higher incidence in the pediatric population. Anti-seizure medication (ASM) may produce adverse drug reactions (ADRs) with an elevated frequency and a high severity. Thus, the objective of the present study was to analyze, through intensive pharmacovigilance over 112 months, the ADRs produced by valproic acid (VPA), oxcarbazepine (OXC), phenytoin (PHT), and levetiracetam (LEV), among others, administered to monotherapy or polytherapy for Mexican hospitalized pediatric epilepsy patients. A total of 1034 patients were interviewed; 315 met the inclusion criteria, 211 patients presented ADRs, and 104 did not. A total of 548 ASM-ADRs were identified, and VPA, LEV, and PHT were the main culprit drugs. The most frequent ADRs were drowsiness, irritability, and thrombocytopenia, and the main systems affected were hematologic, nervous, and dermatologic. LEV and OXC caused more nonsevere ADRs, and PHT caused more severe ADRs. The risk analysis showed an association between belonging to the younger groups and polytherapy with ADR presence and between polytherapy and malnutrition with severe ADRs. In addition, most of the severe ADRs were preventable, and most of the nonsevere ADRs were nonpreventable.
Keywords: adverse drug reactions; anti-seizure medication; epilepsy; generalized estimating equations; levetiracetam; logistic regression; phenytoin; risk factors; valproic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Adverse drug reactions associated with six commonly used antiepileptic drugs in southern China from 2003 to 2015.BMC Pharmacol Toxicol. 2019 Jan 14;20(1):7. doi: 10.1186/s40360-019-0285-y. BMC Pharmacol Toxicol. 2019. PMID: 30642405 Free PMC article.
-
Analysis of antiseizure drug-related adverse reactions from the electronic health record using the common data model.Epilepsia. 2020 Apr;61(4):610-616. doi: 10.1111/epi.16472. Epub 2020 Mar 12. Epilepsia. 2020. PMID: 32162687
-
Pharmacovigilance in Pediatric Patients with Epilepsy Using Antiepileptic Drugs.Int J Environ Res Public Health. 2022 Apr 8;19(8):4509. doi: 10.3390/ijerph19084509. Int J Environ Res Public Health. 2022. PMID: 35457375 Free PMC article.
-
Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy.Epilepsy Behav. 2017 Nov;76:24-31. doi: 10.1016/j.yebeh.2017.08.039. Epub 2017 Sep 18. Epilepsy Behav. 2017. PMID: 28931473 Review.
-
Effectiveness of antiseizure medications therapy in preventing seizures in brain injury patients: A network meta-analysis.Front Pharmacol. 2022 Sep 15;13:1001363. doi: 10.3389/fphar.2022.1001363. eCollection 2022. Front Pharmacol. 2022. PMID: 36188582 Free PMC article.
Cited by
-
Monitoring of the trough concentration of valproic acid in pediatric epilepsy patients: a machine learning-based ensemble model.Front Pharmacol. 2024 Dec 18;15:1521932. doi: 10.3389/fphar.2024.1521932. eCollection 2024. Front Pharmacol. 2024. PMID: 39744128 Free PMC article.
-
[Cognition in pediatric patients with diagnosis of epilepsy].Rev Med Inst Mex Seguro Soc. 2024 May 6;62(3):1-6. doi: 10.5281/zenodo.10998749. Rev Med Inst Mex Seguro Soc. 2024. PMID: 39528273 Free PMC article. Spanish.
References
-
- Cruz-Cruz M.D.R., Gallardo-Elías J., Paredes-Solís S., Legorreta-Soberanis J., Flores-Moreno M., Andersson N. Factores asociados a epilepsia en niños en Mexico: Un estudio caso-control [Factors associated with epilepsy in children in Mexico: A case-control study] Bol. Med. Hosp. Infant Mex. 2017;74:334–340. doi: 10.1016/j.bmhimx.2017.05.006. - DOI - PubMed
-
- Izquierdo A.Y. Crisis convulsivas. Concepto, clasificación y etiología. Emergencias. 2005;17:568–573.
-
- Stafstrom C.E. Pathophysiological mechanisms of seizures and epilepsy: A primer. In: Rho J.M., Sankar R., Stafstrom C.E., editors. Epilepsy: Mechanisms, Models, and Translational Perspectives. 1st ed. CRC Press; New York, NY, USA: 2010. pp. 3–19.
-
- WHO, World Health Organization Epilepsy. 2023. [(accessed on 5 July 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

