Early-Term Neonates Demonstrate a Higher Likelihood of Requiring Phototherapy Compared to Those Born Full-Term
- PMID: 38002910
- PMCID: PMC10670379
- DOI: 10.3390/children10111819
Early-Term Neonates Demonstrate a Higher Likelihood of Requiring Phototherapy Compared to Those Born Full-Term
Abstract
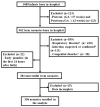
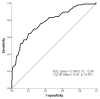
Early-term neonates (with a gestational age (GA) of 37 and 0/7 weeks to 38 and 6/7 weeks) face higher morbidities, including respiratory and neurodevelopmental issues, than full-term (39 and 0/7 weeks to 40 and 6/7 weeks) infants. This study explores whether hyperbilirubinemia necessitating phototherapy also differs between these groups. A retrospective study was conducted on neonates born from January 2021-June 2022, excluding those with specific conditions. Evaluated factors included GA, birth weight, bilirubin levels, glucose-6-phosphate dehydrogenase (G6PD) deficiency, and feeding type, with phototherapy given as per AAP guidelines. Of 1085 neonates, 356 met the criteria. When stratifying the neonates based on the need for phototherapy, a higher proportion of early-term neonates required phototherapy compared to full-term (p < 0.05). After factoring in various risks (GA; birth weight; gender; feeding type; G6PD deficiency; transcutaneous bilirubin levels at 24 h and 24-48 h postpartum; maternal diabetes; and the presence of caput succedaneum or cephalohematoma), early-term neonates were more likely to need phototherapy than full-term babies (OR: 2.15, 95% CI: 1.21 to 3.80). The optimal cut-off for transcutaneous bilirubin levels 24-48 h postpartum that were used to predict phototherapy need was 9.85 mg/dl. In conclusion, early-term neonates are at a greater risk for developing jaundice and requiring phototherapy than full-term neonates. Monitoring bilirubin 24-48 h postpartum enhances early prediction and intervention.
Keywords: early term; full term; hyperbilirubinemia; phototherapy; transcutaneous bilirubin.
Conflict of interest statement
The authors declare no conflict of interest. The funding sources were not involved in the study’s design, data collection, analysis, or interpretation; the composition of the manuscript; or the decision to submit the results for publication.
Figures
Similar articles
-
Risk Factors Predicting the Need for Phototherapy in Glucose 6 Phosphate Dehydrogenase-Deficient Infants in a Large Retrospective Cohort Study.Neonatology. 2022;119(4):494-500. doi: 10.1159/000524966. Epub 2022 Jun 14. Neonatology. 2022. PMID: 35700699
-
Control of hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns using an inhibitor of bilirubin production, Sn-mesoporphyrin.Pediatrics. 1998 May;101(5):E1. doi: 10.1542/peds.101.5.e1. Pediatrics. 1998. PMID: 9565434 Clinical Trial.
-
Sunlight for the prevention and treatment of hyperbilirubinemia in term and late preterm neonates.Cochrane Database Syst Rev. 2021 Jul 6;7(7):CD013277. doi: 10.1002/14651858.CD013277.pub2. Cochrane Database Syst Rev. 2021. PMID: 34228352 Free PMC article.
-
Transcutaneous bilirubin level to predict hyperbilirubinemia in preterm neonates.F1000Res. 2020 Apr 28;9:300. doi: 10.12688/f1000research.22264.2. eCollection 2020. F1000Res. 2020. PMID: 33014346 Free PMC article.
-
Cochrane Review: Prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants.Evid Based Child Health. 2013 Jan;8(1):204-49. doi: 10.1002/ebch.1898. Evid Based Child Health. 2013. PMID: 23878128 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

