Genome-Wide Association Analysis across Endophenotypes in Alzheimer's Disease: Main Effects and Disease Stage-Specific Interactions
- PMID: 38002954
- PMCID: PMC10671827
- DOI: 10.3390/genes14112010
Genome-Wide Association Analysis across Endophenotypes in Alzheimer's Disease: Main Effects and Disease Stage-Specific Interactions
Abstract
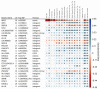
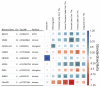
The underlying genetic susceptibility for Alzheimer's disease (AD) is not yet fully understood. The heterogeneous nature of the disease challenges genetic association studies. Endophenotype approaches can help to address this challenge by more direct interrogation of biological traits related to the disease. AD endophenotypes based on amyloid-β, tau, and neurodegeneration (A/T/N) biomarkers and cognitive performance were selected from the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort (N = 1565). A genome-wide association study (GWAS) of quantitative phenotypes was performed using an SNP main effect and an SNP by Diagnosis interaction (SNP × DX) model to identify disease stage-specific genetic effects. Nine loci were identified as study-wide significant with one or more A/T/N endophenotypes in the main effect model, as well as additional findings significantly associated with cognitive measures. These nine loci include SNPs in or near the genes APOE, SRSF10, HLA-DQB1, XKR3, and KIAA1671. The SNP × DX model identified three study-wide significant genetic loci (BACH2, EP300, and PACRG-AS1) with a neuroprotective effect in later AD stage endophenotypes. An endophenotype approach identified novel genetic associations and provided insight into the molecular mechanisms underlying the genetic associations that may otherwise be missed using conventional case-control study designs.
Keywords: APOE; FDG-PET; GWAS; amyloid-PET; cerebrospinal fluid biomarkers; endophenotype; genetic interaction; genetics; magnetic resonance imaging.
Conflict of interest statement
Andrew Saykin receives support from multiple NIH grants (P30 AG010133, P30 AG072976, R01 AG019771, R01 AG057739, U19 AG024904, R01 LM013463, R01 AG068193, T32 AG071444, U01 AG068057, U01 AG072177, and U19 AG074879). He has also received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in kind contribution of PET tracer precursor); Bayer Oncology (Scientific Advisory Board); Eisai (Scientific Advisory Board); Siemens Medical Solutions USA, Inc. (Dementia Advisory Board); NIH NHLBI (MESA Observational Study Monitoring Board); Springer-Nature Publishing (Editorial Office Support as Editor-in-Chief, Brain Imaging and Behavior). Li Shen has received additional funding paid to the University of Pennsylvania from NSF IIS 1622526/1827472, NSF IIS 1837964, NIH RF1 AG063481, NIH R01 LM013463, NIH U01 AG068057, NIH R01 AG058854, NIH RF1 AG068191, NIH R01 AG071470, NIH R01 EB022574. Li Shen has served as consultant on NIH grant R24 EB029173 from the University of Massachusetts. Tatiana Foroud acts as a consultant for NIH-funded centers and other infrastructure grants and receives funding for travel expenses from the Michael J. Fox Foundation and from academic institutions on which she serves as advisor. Sujuan Gao has served as an unpaid board and committee member on the data safety monitoring board for R01 AG058586, 18G-MC-LMDC, R01 HL151951, and paid data safety monitoring board member for R01 AG061898. Shannon Risacher served as Communications Chair, unpaid, for the Alzheimer’s Association AWARE PIA, as well as received travel funding for the Charleston Conference on Alzheimer’s Disease. She also has equity interest in Eli Lilly (<$10000), a company that may potentially benefit in the research results of this study. Thea Rosewood and Kwangsik Nho have no interests to declare. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Knowledge-driven binning approach for rare variant association analysis: application to neuroimaging biomarkers in Alzheimer's disease.BMC Med Inform Decis Mak. 2017 May 18;17(Suppl 1):61. doi: 10.1186/s12911-017-0454-0. BMC Med Inform Decis Mak. 2017. PMID: 28539126 Free PMC article.
-
Role of Imaging Genetics in Alzheimer's Disease: A Systematic Review and Current Update.CNS Neurol Disord Drug Targets. 2024;23(9):1143-1156. doi: 10.2174/0118715273264879231027070642. CNS Neurol Disord Drug Targets. 2024. PMID: 38243986
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
Association analysis of rare variants near the APOE region with CSF and neuroimaging biomarkers of Alzheimer's disease.BMC Med Genomics. 2017 May 24;10(Suppl 1):29. doi: 10.1186/s12920-017-0267-0. BMC Med Genomics. 2017. PMID: 28589856 Free PMC article.
-
Impact of diabetes on the progression of Alzheimer's disease via trajectories of amyloid-tau-neurodegeneration (ATN) biomarkers.J Nutr Health Aging. 2025 Feb;29(2):100444. doi: 10.1016/j.jnha.2024.100444. Epub 2024 Dec 10. J Nutr Health Aging. 2025. PMID: 39662155 Free PMC article.
Cited by
-
Pathway enrichment in genome-wide analysis of longitudinal Alzheimer's disease biomarker endophenotypes.Alzheimers Dement. 2024 Dec;20(12):8639-8650. doi: 10.1002/alz.14308. Epub 2024 Oct 23. Alzheimers Dement. 2024. PMID: 39440837 Free PMC article.
-
miR-137: A therapeutic candidate or a key molecular regulator in Alzheimer's disease?J Alzheimers Dis Rep. 2025 Jun 25;9:25424823251352166. doi: 10.1177/25424823251352166. eCollection 2025 Jan-Dec. J Alzheimers Dis Rep. 2025. PMID: 40575117 Free PMC article. Review.
References
-
- Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. - DOI - PMC - PubMed
-
- Saykin A.J., Shen L., Yao X., Kim S., Nho K., Risacher S.L., Ramanan V.K., Foroud T.M., Faber K.M., Sarwar N., et al. Genetic Studies of Quantitative MCI and AD Phenotypes in ADNI: Progress, Opportunities, and Plans. Alzheimers Dement. 2015;11:792–814. doi: 10.1016/j.jalz.2015.05.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AG071444/AG/NIA NIH HHS/United States
- U01 AG032984/AG/NIA NIH HHS/United States
- U01 AG068057/AG/NIA NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- R01 AG061788/AG/NIA NIH HHS/United States
- P30 AG072976/AG/NIA NIH HHS/United States
- R03 AG063250/AG/NIA NIH HHS/United States
- R01 LM013463/LM/NLM NIH HHS/United States
- R01 AG019771/AG/NIA NIH HHS/United States
- RC2 AG036528/AG/NIA NIH HHS/United States
- P50 GM115318/GM/NIGMS NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- U19 AG074879/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- K01 AG049050/AG/NIA NIH HHS/United States
- R01 AG057739/AG/NIA NIH HHS/United States
- R01 AG068193/AG/NIA NIH HHS/United States
- R01 LM012535/LM/NLM NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

