Genome-Wide Association Study of Beta-Blocker Survival Benefit in Black and White Patients with Heart Failure with Reduced Ejection Fraction
- PMID: 38002962
- PMCID: PMC10671316
- DOI: 10.3390/genes14112019
Genome-Wide Association Study of Beta-Blocker Survival Benefit in Black and White Patients with Heart Failure with Reduced Ejection Fraction
Abstract
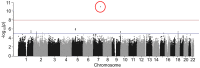
In patients with heart failure with reduced ejection fraction (HFrEF), individual responses to beta-blockers vary. Candidate gene pharmacogenetic studies yielded significant but inconsistent results, and they may have missed important associations. Our objective was to use an unbiased genome-wide association study (GWAS) to identify loci influencing beta-blocker survival benefit in HFrEF patients. Genetic variant × beta-blocker exposure interactions were tested in Cox proportional hazards models for all-cause mortality stratified by self-identified race. The models were adjusted for clinical risk factors and propensity scores. A prospective HFrEF registry (469 black and 459 white patients) was used for discovery, and linkage disequilibrium (LD) clumped variants with a beta-blocker interaction of p < 5 × 10-5, were tested for Bonferroni-corrected validation in a multicenter HFrEF clinical trial (288 black and 579 white patients). A total of 229 and 18 variants in black and white HFrEF patients, respectively, had interactions with beta-blocker exposure at p < 5 × 10-5 upon discovery. After LD-clumping, 100 variants and 4 variants in the black and white patients, respectively, remained for validation but none reached statistical significance. In conclusion, genetic variants of potential interest were identified in a discovery-based GWAS of beta-blocker survival benefit in HFrEF patients, but none were validated in an independent dataset. Larger cohorts or alternative approaches, such as polygenic scores, are needed.
Keywords: beta-blocker; genome-wide association study; heart failure with reduced ejection fraction; survival.
Conflict of interest statement
Dr. Lanfear is a consultant for Amgen, Janssen, Ortho Diagnostics, and Duke Clinical Research Institute (Novartis); has participated in clinical trials from Amgen, Bayer, and Janssen; and has submitted a provisional patent request for the β-blocker polygenic score. Dr. Luzum is a consultant for Ariel Precision Medicine. The other authors report no conflict.
Figures


References
-
- Maddox T.M., Januzzi J.L., Allen L.A., Breathett K., Butler J., Davis L.L., Fonarow G.C., Ibrahim N.E., Lindenfeld J., Masoudi F.A., et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021;77:772–810. doi: 10.1016/j.jacc.2020.11.022. - DOI - PubMed
-
- Packer M., Bristow M.R., Cohn J.N., Colucci W.S., Fowler M.B., Gilbert E.M., Shusterman N.H. The Effect of Carvedilol on Morbidity and Mortality in Patients with Chronic Heart Failure. U.S. Carvedilol Heart Failure Study Group. N. Engl. J. Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL141845/NH/NIH HHS/United States
- L30HL110279/NH/NIH HHS/United States
- R01 DK113003/DK/NIDDK NIH HHS/United States
- R01DK064695/NH/NIH HHS/United States
- R01 HL132154/HL/NHLBI NIH HHS/United States
- K08 HL146990/HL/NHLBI NIH HHS/United States
- P50 MD017351/MD/NIMHD NIH HHS/United States
- R01DK113003/NH/NIH HHS/United States
- P01HL074237/NH/NIH HHS/United States
- R01 HL118267/HL/NHLBI NIH HHS/United States
- R01AI079139/NH/NIH HHS/United States
- F32 HL162231/HL/NHLBI NIH HHS/United States
- R01 HL103871/HL/NHLBI NIH HHS/United States
- K08HL146990/NH/NIH HHS/United States
- R01 AI079139/AI/NIAID NIH HHS/United States
- R01 HL141845/HL/NHLBI NIH HHS/United States
- L30 HL110279/HL/NHLBI NIH HHS/United States
- P50MD017351/NH/NIH HHS/United States
- P01 HL074237/HL/NHLBI NIH HHS/United States
- F32HL162231/NH/NIH HHS/United States
- R01HL118267/NH/NIH HHS/United States
- R01HL132154/NH/NIH HHS/United States
- R01 DK064695/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

