Study of Transcriptomic Analysis of Yak (Bos grunniens) and Cattle (Bos taurus) Pulmonary Artery Smooth Muscle Cells under Oxygen Concentration Gradients and Differences in Their Lung Histology and Expression of Pyruvate Dehydrogenase Kinase 1-Related Factors
- PMID: 38003068
- PMCID: PMC10668684
- DOI: 10.3390/ani13223450
Study of Transcriptomic Analysis of Yak (Bos grunniens) and Cattle (Bos taurus) Pulmonary Artery Smooth Muscle Cells under Oxygen Concentration Gradients and Differences in Their Lung Histology and Expression of Pyruvate Dehydrogenase Kinase 1-Related Factors
Abstract
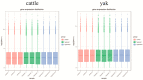
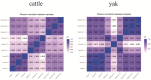
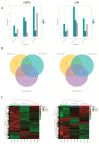
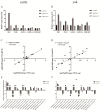
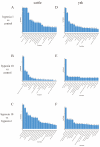
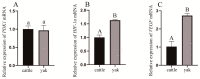
The aim of this study was to investigate the molecular mechanisms by which hypoxia affects the biological behavior of yak PASMCs, the changes in the histological structure of yak and cattle lungs, and the relationships and regulatory roles that exist regarding the differences in the distribution and expression of PDK1 and its hypoxia-associated factors screened for their role in the adaptation of yak lungs to the plateau hypoxic environment. The results showed that, at the level of transcriptome sequencing, the molecular regulatory mechanisms of the HIF-1 signaling pathway, glucose metabolism pathway, and related factors (HK2/PGK1/ENO1/ENO3/ALDOC/ALDOA) may be closely related to the adaptation of yaks to the hypoxic environment of the plateau; at the tissue level, the presence of filled alveoli and semi-filled alveoli, thicker alveolar septa and basement membranes, a large number of erythrocytes, capillary distribution, and collagen fibers accounted for all levels of fine bronchioles in the lungs of yaks as compared to cattle. A higher percentage of goblet cells was found in the fine bronchioles of yaks, and PDK1, HIF-1α, and VEGF were predominantly distributed and expressed in the monolayers of ciliated columnar epithelium in the branches of the terminal fine bronchioles of yak and cattle lungs, with a small amount of it distributed in the alveolar septa; at the molecular level, the differences in PDK1 mRNA relative expression in the lungs of adult yaks and cattle were not significant (p > 0.05), the differences in HIF-1α and VEGF mRNA relative expression were significant (p < 0.05), and the expression of PDK1 and HIF-1α proteins in adult yaks was stronger than that in adult cattle. PDK1 and HIF-1α proteins were more strongly expressed in adult yaks than in adult cattle, and the difference was highly significant (p < 0.01); the relative expression of VEGF proteins was not significantly different between adult yaks and cattle (p > 0.05). The possible regulatory relationship between the above results and the adaptation of yak lungs to the plateau hypoxic environment paves the way for the regulatory mechanisms of PDK1, HIF-1α, and VEGF, and provides basic information for studying the mechanism of hypoxic adaptation of yaks in the plateau. At the same time, it provides a reference for human hypoxia adaptation and a target for the prevention and treatment of plateau diseases in humans and plateau animals.
Keywords: PASMCs; cattle; different oxygen concentrations; expression distribution; hypoxic differential factor; lung histology; transcriptomic analysis; yak.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







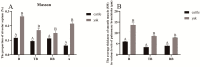
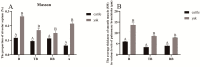





Similar articles
-
Study on the Regulatory Mechanism of the PDK1-Mediated TGF-β/Smad Signaling Pathway in Hypoxia-Induced Yak Lungs.Animals (Basel). 2024 Aug 21;14(16):2422. doi: 10.3390/ani14162422. Animals (Basel). 2024. PMID: 39199957 Free PMC article.
-
Hypoxia-Induced Differences in the Expression of Pyruvate Dehydrogenase Kinase 1-Related Factors in the Renal Tissues and Renal Interstitial Fibroblast-like Cells of Yak (Bos Grunniens).Animals (Basel). 2024 Oct 29;14(21):3110. doi: 10.3390/ani14213110. Animals (Basel). 2024. PMID: 39518833 Free PMC article.
-
Differential expression of HIF-1α and its hypoxia-related inducers in the spleens of plateau yaks and plain yellow cattle.Histol Histopathol. 2025 Feb;40(2):225-235. doi: 10.14670/HH-18-768. Epub 2024 May 28. Histol Histopathol. 2025. PMID: 38864176
-
Adaptation Mechanisms of Yak (Bos grunniens) to High-Altitude Environmental Stress.Animals (Basel). 2021 Aug 9;11(8):2344. doi: 10.3390/ani11082344. Animals (Basel). 2021. PMID: 34438801 Free PMC article. Review.
-
Yaks Are Dependent on Gut Microbiota for Survival in the Environment of the Qinghai Tibet Plateau.Microorganisms. 2024 May 31;12(6):1122. doi: 10.3390/microorganisms12061122. Microorganisms. 2024. PMID: 38930503 Free PMC article. Review.
Cited by
-
Metabolic Plasticity and Transcriptomic Reprogramming Orchestrate Hypoxia Adaptation in Yak.Animals (Basel). 2025 Jul 15;15(14):2084. doi: 10.3390/ani15142084. Animals (Basel). 2025. PMID: 40723548 Free PMC article.
-
Transcriptomic Profiling of Hypoxia-Adaptive Responses in Tibetan Goat Fibroblasts.Animals (Basel). 2025 May 13;15(10):1407. doi: 10.3390/ani15101407. Animals (Basel). 2025. PMID: 40427283 Free PMC article.
-
Changes of collagen content in lung tissues of plateau yak and its mechanism of adaptation to hypoxia.PeerJ. 2024 Oct 1;12:e18250. doi: 10.7717/peerj.18250. eCollection 2024. PeerJ. 2024. PMID: 39372716 Free PMC article.
-
Study on the Regulatory Mechanism of the PDK1-Mediated TGF-β/Smad Signaling Pathway in Hypoxia-Induced Yak Lungs.Animals (Basel). 2024 Aug 21;14(16):2422. doi: 10.3390/ani14162422. Animals (Basel). 2024. PMID: 39199957 Free PMC article.
References
-
- Xin J.W., Chai Z.X., Zhang C.F., Zhang Q., Zhu Y., Cao H.W., Yangji C., Chen X.Y., Jiang H., Zhong J.C., et al. Differences in proteomic profiles between yak and three cattle strains provide insights into molecular mechanisms underlying high-altitude adaptation. J. Anim. Physiol. Anim. Nutr. 2022;106:485–493. doi: 10.1111/jpn.13629. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

