Skeletal Morphogenesis and Anomalies in Gilthead Seabream: A Comprehensive Review
- PMID: 38003219
- PMCID: PMC10671147
- DOI: 10.3390/ijms242216030
Skeletal Morphogenesis and Anomalies in Gilthead Seabream: A Comprehensive Review
Abstract
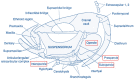
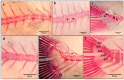
The gilthead seabream, one of the most important species in Mediterranean aquaculture, with an increasing status of exploitation in terms of production volume and aquafarming technologies, has become an important research topic over the years. The accumulation of knowledge from several studies conducted during recent decades on their functional and biological characteristics has significantly improved their aquacultural aspects, namely their reproductive success, survival, and growth. Despite the remarkable progress in the aquaculture industry, hatchery conditions are still far from ideal, resulting in frequent abnormalities at the beginning of intensive culture, entailing significant economic losses. Those deformities are induced during the embryonic and post-embryonic periods of life, and their development is still poorly understood. In the present review, we created a comprehensive synthesis that covers the various aspects of skeletal morphogenesis and anomalies in the gilthead seabream, highlighting the genetic, environmental, and nutritional factors contributing to bone deformities and emphasized the potential of the gilthead seabream as a model organism for understanding bone morphogenesis in both aquaculture and translational biological research. This review article addresses the existing lack in the literature regarding gilthead seabream bone deformities, as there are currently no comprehensive reviews on this subject.
Keywords: bone deformities; craniofacial anomalies; genes; gilthead seabream; models; ossification pattern; skeletal morphogenesis; spinal deformities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Abbate F., Guerrera M.C., Levanti M., Laurà R., Aragona M., Mhalhel K., Montalbano G., Germanà A. Morphological Characteristics of the Blackspot Seabream (Pagellus bogaraveo) Tongue: A Structural and Immunohistochemical Study. Anat. Histol. Embryol. 2022;51:103–111. doi: 10.1111/ahe.12769. - DOI - PMC - PubMed
-
- Dellacqua Z., Di Biagio C., Costa C., Pousão-Ferreira P., Ribeiro L., Barata M., Gavaia P.J., Mattei F., Fabris A., Izquierdo M., et al. Distinguishing the Effects of Water Volumes versus Stocking Densities on the Skeletal Quality during the Pre-Ongrowing Phase of Gilthead Seabream (Sparus aurata) Animals. 2023;13:557. doi: 10.3390/ani13040557. - DOI - PMC - PubMed
-
- Mhalhel K., Montalbano G., Giurdanella G., Abbate F., Laurà R., Guerrera M.C., Germanà A., Levanti M. Histological and Immunohistochemical Study of Gilthead Seabream Tongue from the Early Stage of Development: TRPV4 Potential Roles. Ann. Anat. 2022;244:151985. doi: 10.1016/j.aanat.2022.151985. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

