Formoterol Exerts Anti-Cancer Effects Modulating Oxidative Stress and Epithelial-Mesenchymal Transition Processes in Cigarette Smoke Extract Exposed Lung Adenocarcinoma Cells
- PMID: 38003276
- PMCID: PMC10671675
- DOI: 10.3390/ijms242216088
Formoterol Exerts Anti-Cancer Effects Modulating Oxidative Stress and Epithelial-Mesenchymal Transition Processes in Cigarette Smoke Extract Exposed Lung Adenocarcinoma Cells
Abstract
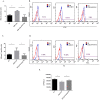
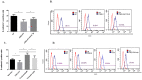
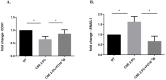
Lung cancer frequently affects patients with Chronic Obstructive Pulmonary Disease (COPD). Cigarette smoke (CS) fosters cancer progression by increasing oxidative stress and by modulating epithelial-mesenchymal transition (EMT) processes in cancer cells. Formoterol (FO), a long-acting β2-agonist widely used for the treatment of COPD, exerts antioxidant activities. This study explored in a lung adenocarcinoma cell line (A549) whether FO counteracted the effects of cigarette smoke extract (CSE) relative to oxidative stress, inflammation, EMT processes, and cell migration and proliferation. A549 was stimulated with CSE and FO, ROS were evaluated by flow-cytometry and by nanostructured electrochemical sensor, EMT markers were evaluated by flow-cytometry and Real-Time PCR, IL-8 was evaluated by ELISA, cell migration was assessed by scratch and phalloidin test, and cell proliferation was assessed by clonogenic assay. CSE significantly increased the production of ROS, IL-8 release, cell migration and proliferation, and SNAIL1 expression but significantly decreased E-cadherin expression. FO reverted all these phenomena in CSE-stimulated A549 cells. The present study provides intriguing evidence that FO may exert anti-cancer effects by reverting oxidative stress, inflammation, and EMT markers induced by CS. These findings must be validated in future clinical studies to support FO as a valuable add-on treatment for lung cancer management.
Keywords: EMT; cigarette smoke; formoterol; inflammation; lung cancer; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Formoterol and fluticasone propionate combination improves histone deacetylation and anti-inflammatory activities in bronchial epithelial cells exposed to cigarette smoke.Biochim Biophys Acta Mol Basis Dis. 2017 Jul;1863(7):1718-1727. doi: 10.1016/j.bbadis.2017.05.003. Epub 2017 May 5. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28483577
-
Oxidative Stress and Epithelial-Mesenchymal Transition: The Impact of Ubiquitin C-terminal Hydrolase L1 in Cigarette Smoke-Induced COPD.Lung. 2025 Feb 25;203(1):36. doi: 10.1007/s00408-025-00790-x. Lung. 2025. PMID: 40000498
-
Conditioned Media of Adipose-Derived Stem Cells Suppresses Sidestream Cigarette Smoke Extract Induced Cell Death and Epithelial-Mesenchymal Transition in Lung Epithelial Cells.Int J Mol Sci. 2021 Nov 8;22(21):12069. doi: 10.3390/ijms222112069. Int J Mol Sci. 2021. PMID: 34769496 Free PMC article.
-
The effects of epithelial-mesenchymal transitions in COPD induced by cigarette smoke: an update.Respir Res. 2022 Aug 31;23(1):225. doi: 10.1186/s12931-022-02153-z. Respir Res. 2022. PMID: 36045410 Free PMC article. Review.
-
Cellular and Molecular Signatures of Oxidative Stress in Bronchial Epithelial Cell Models Injured by Cigarette Smoke Extract.Int J Mol Sci. 2022 Feb 4;23(3):1770. doi: 10.3390/ijms23031770. Int J Mol Sci. 2022. PMID: 35163691 Free PMC article. Review.
Cited by
-
Dissecting the pathogenic effects of smoking in blood DNA methylation on allergic diseases.World Allergy Organ J. 2024 Nov 21;17(12):100995. doi: 10.1016/j.waojou.2024.100995. eCollection 2024 Dec. World Allergy Organ J. 2024. PMID: 39640897 Free PMC article.
-
Oxidative Stress and Age-Related Tumors.Antioxidants (Basel). 2024 Sep 13;13(9):1109. doi: 10.3390/antiox13091109. Antioxidants (Basel). 2024. PMID: 39334768 Free PMC article. Review.
-
Targeting Non-Eosinophilic Immunological Pathways in COPD and AECOPD: Current Insights and Therapeutic Strategies.Int J Chron Obstruct Pulmon Dis. 2025 Mar 5;20:511-532. doi: 10.2147/COPD.S506616. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40066199 Free PMC article. Review.
-
Shake It Up Baby Now: The Changing Focus on TWIST1 and Epithelial to Mesenchymal Transition in Cancer and Other Diseases.Int J Mol Sci. 2023 Dec 16;24(24):17539. doi: 10.3390/ijms242417539. Int J Mol Sci. 2023. PMID: 38139368 Free PMC article. Review.
-
Emerging trends and hotspots in chronic obstructive pulmonary disease and oxidative stress: a bibliometric and visualized analysis from 2010 to 2024.J Thorac Dis. 2025 Mar 31;17(3):1228-1248. doi: 10.21037/jtd-24-1679. Epub 2025 Mar 26. J Thorac Dis. 2025. PMID: 40223997 Free PMC article.
References
-
- Wilson D.O., Weissfeld J.L., Balkan A., Schragin J.G., Fuhrman C.R., Fisher S.N., Wilson J., Leader J.K., Siegfried J.M., Shapiro S.D., et al. Association of Radiographic Emphysema and Airflow Obstruction with Lung Cancer. Am. J. Respir. Crit. Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. - DOI - PMC - PubMed
-
- de Torres J.P., Marín J.M., Casanova C., Cote C., Carrizo S., Cordoba-Lanus E., Baz-Dávila R., Zulueta J.J., Aguirre-Jaime A., Saetta M., et al. Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2011;184:913–919. doi: 10.1164/rccm.201103-0430OC. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

