Effect of SARS-CoV-2 Infection and BNT162b2 Vaccination on the mRNA Expression of Genes Associated with Angiogenesis
- PMID: 38003287
- PMCID: PMC10671623
- DOI: 10.3390/ijms242216094
Effect of SARS-CoV-2 Infection and BNT162b2 Vaccination on the mRNA Expression of Genes Associated with Angiogenesis
Abstract
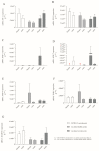
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), discovered in December 2019 in Wuhan, China, caused the coronavirus disease 2019 (COVID-19). Due to the rate of spread of this virus, the World Health Organization, in March 2020, recognised COVID-19 as a worldwide pandemic. The disease is multisystemic with varying degrees of severity. Unfortunately, despite intensive research, the molecular changes caused by SARS-CoV-2 remain unclear. Mechanisms affected by the virus infection include endothelial dysfunction and angiogenesis. Similarly, the vaccines developed so far affect the process of angiogenesis, contributing to the development of undesirable effects on part of the cardiovascular system. The presented research aimed to investigate the impact of the SARS-CoV-2 infection and the Pfizer Comirnaty vaccine (BNT162b2) on the molecular aspect of angiogenesis. We found that convalescents vaccinated with one dose of BNT162b2 were characterised by higher MMP-7 (metalloproteinases 7) expression than non-vaccinated convalescents and healthy volunteers vaccinated with one dose of BNT162b2. Moreover, non-vaccinated convalescents showed increased mRNA expression of ADAMTS1 (ADAM metallopeptidase with thrombospondin type 1 motif 1) compared to healthy volunteers vaccinated with one dose of BNT162b2. In addition, we showed significant sex differences in the expression of MMP-7. In conclusion, the results of our study suggest a significant impact of SARS-CoV-2 infection and vaccination on the course of angiogenesis at the molecular level.
Keywords: BNT162b2 vaccination; COVID-19; angiogenesis; mRNA expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Protection from previous natural infection compared with mRNA vaccination against SARS-CoV-2 infection and severe COVID-19 in Qatar: a retrospective cohort study.Lancet Microbe. 2022 Dec;3(12):e944-e955. doi: 10.1016/S2666-5247(22)00287-7. Epub 2022 Nov 11. Lancet Microbe. 2022. PMID: 36375482 Free PMC article.
-
Analysis of COVID-19 Incidence and Severity Among Adults Vaccinated With 2-Dose mRNA COVID-19 or Inactivated SARS-CoV-2 Vaccines With and Without Boosters in Singapore.JAMA Netw Open. 2022 Aug 1;5(8):e2228900. doi: 10.1001/jamanetworkopen.2022.28900. JAMA Netw Open. 2022. PMID: 36018588 Free PMC article.
-
Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study.Lancet Infect Dis. 2022 Jan;22(1):56-63. doi: 10.1016/S1473-3099(21)00479-5. Epub 2021 Sep 9. Lancet Infect Dis. 2022. PMID: 34509185 Free PMC article.
-
Postvaccination infections among staff of a tertiary care hospital after vaccination with severe acute respiratory syndrome coronavirus 2 vector and mRNA-based vaccines.Clin Microbiol Infect. 2022 Apr;28(4):596-601. doi: 10.1016/j.cmi.2021.11.023. Epub 2021 Dec 13. Clin Microbiol Infect. 2022. PMID: 34915073 Free PMC article.
-
MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature.J Neurol. 2022 Oct;269(10):5198-5212. doi: 10.1007/s00415-022-11194-9. Epub 2022 Jun 23. J Neurol. 2022. PMID: 35737110 Free PMC article. Review.
Cited by
-
Molecular Research on Coronavirus: Pathogenic Mechanisms, Antiviral Drugs, and New Vaccines.Int J Mol Sci. 2024 Jun 4;25(11):6172. doi: 10.3390/ijms25116172. Int J Mol Sci. 2024. PMID: 38892360 Free PMC article.
References
-
- National Center for Biotechnology Information (NCBI) National Library of Medicine. National Institutes of Health. Department of Health and Human Services Variation Viewer. [(accessed on 1 September 2023)]; Available online: https://www.ncbi.nlm.nih.gov/variation/view/
-
- Awwad A.A., Elhay O.M.A., Rabie M.M., Awad A.E., Kotb F.M., Maghraby H.M., Eldamarawy R.H., Dawood Y.M., Balat M.I., Hasan A.I., et al. Impact of Systemic Diseases on Olfactory Function in COVID-19 Infected Patients. Int. J. Gen. Med. 2022;15:5681–5691. doi: 10.2147/IJGM.S355974. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

