Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review
- PMID: 38003311
- PMCID: PMC10671333
- DOI: 10.3390/ijms242216123
Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review
Abstract
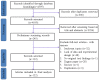
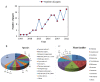
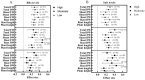
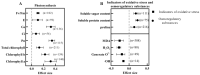
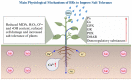
Brassinosteroids (BRs), the sixth major phytohormone, can regulate plant salt tolerance. Many studies have been conducted to investigate the effects of BRs on plant salt tolerance, generating a large amount of research data. However, a meta-analysis on regulating plant salt tolerance by BRs has not been reported. Therefore, this study conducted a meta-analysis of 132 studies to elucidate the most critical physiological mechanisms by which BRs regulate salt tolerance in plants from a higher dimension and analyze the best ways to apply BRs. The results showed that exogenous BRs significantly increased germination, plant height, root length, and biomass (total dry weight was the largest) of plants under salt stress. There was no significant difference between seed soaking and foliar spraying. However, the medium method (germination stage) and stem application (seedling stage) may be more effective in improving plant salt tolerance. BRs only inhibit germination in Solanaceae. BRs (2 μM), seed soaking for 12 h, and simultaneous treatment with salt stress had the highest germination rate. At the seedling stage, the activity of Brassinolide (C28H48O6) was higher than that of Homobrassinolide (C29H50O6), and post-treatment, BRs (0.02 μM) was the best solution. BRs are unsuitable for use in the germination stage when Sodium chloride is below 100 mM, and the effect is also weakest in the seedling stage. Exogenous BRs promoted photosynthesis, and antioxidant enzyme activity increased the accumulation of osmoregulatory and antioxidant substances and reduced the content of harmful substances and Na+, thus reducing cell damage and improving plant salt tolerance. BRs induced the most soluble protein, chlorophyll a, stomatal conductance, net photosynthetic rate, Glutathione peroxidase, and root-Ca2+, with BRs causing Ca2+ signals in roots probably constituting the most important reason for improving salt tolerance. BRs first promoted the accumulation of Ca2+ in roots, which increased the content of the above vital substances and enzyme activities through the Ca2+ signaling pathway, improving plant salt tolerance.
Keywords: brassinosteroids; meta-analysis; physiological; salt stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
Crucial Roles of Brassinosteroids in Cell Wall Composition and Structure Across Species: New Insights and Biotechnological Applications.Plant Cell Environ. 2025 Mar;48(3):1751-1767. doi: 10.1111/pce.15258. Epub 2024 Nov 3. Plant Cell Environ. 2025. PMID: 39491539 Free PMC article. Review.
-
Understanding the Brassinosteroid-Dependent Environmental Adaption in Brassicaceae Plants.Plants (Basel). 2025 May 21;14(10):1554. doi: 10.3390/plants14101554. Plants (Basel). 2025. PMID: 40431121 Free PMC article. Review.
References
-
- Matishov G.G., Grigorenko K.S. Causes of salinization of the Gulf of Taganrog. Dokl. Earth Sci. 2017;477:1311–1315. doi: 10.1134/S1028334X17110034. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

