Mucopolysaccharidosis IVA: Current Disease Models and Drawbacks
- PMID: 38003337
- PMCID: PMC10671113
- DOI: 10.3390/ijms242216148
Mucopolysaccharidosis IVA: Current Disease Models and Drawbacks
Abstract
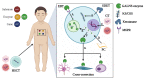
Mucopolysaccharidosis IVA (MPS IVA) is a rare disorder caused by mutations in the N-acetylgalactosamine-6-sulfate-sulfatase (GALNS) encoding gene. GALNS leads to the lysosomal degradation of the glycosaminoglyccreasans keratan sulfate and chondroitin 6-sulfate. Impaired GALNS enzymes result in skeletal and non-skeletal complications in patients. For years, the MPS IVA pathogenesis and the assessment of promising drugs have been evaluated using in vitro (primarily fibroblasts) and in vivo (mainly mouse) models. Even though value information has been raised from those studies, these models have several limitations. For instance, chondrocytes have been well recognized as primary cells affected in MPS IVA and responsible for displaying bone development impairment in MPS IVA patients; nonetheless, only a few investigations have used those cells to evaluate basic and applied concepts. Likewise, current animal models are extensively represented by mice lacking GALNS expression; however, it is well known that MPS IVA mice do not recapitulate the skeletal dysplasia observed in humans, making some comparisons difficult. This manuscript reviews the current in vitro and in vivo MPS IVA models and their drawbacks.
Keywords: chondrocytes; fibroblasts; mouse; mucopolysaccharidosis IVA; rat.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Enzyme replacement in a human model of mucopolysaccharidosis IVA in vitro and its biodistribution in the cartilage of wild type mice.PLoS One. 2010 Aug 16;5(8):e12194. doi: 10.1371/journal.pone.0012194. PLoS One. 2010. PMID: 20808938 Free PMC article.
-
Mucopolysaccharidosis IVA: correlation between genotype, phenotype and keratan sulfate levels.Mol Genet Metab. 2013 Sep-Oct;110(1-2):129-38. doi: 10.1016/j.ymgme.2013.06.008. Epub 2013 Jun 26. Mol Genet Metab. 2013. PMID: 23876334 Free PMC article.
-
Molecular genetics and metabolism, special edition: Diagnosis, diagnosis and prognosis of Mucopolysaccharidosis IVA.Mol Genet Metab. 2018 Sep;125(1-2):18-37. doi: 10.1016/j.ymgme.2018.05.004. Epub 2018 May 15. Mol Genet Metab. 2018. PMID: 29779902 Free PMC article. Review.
-
Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA.Mol Genet Metab. 2013 Sep-Oct;110(1-2):54-64. doi: 10.1016/j.ymgme.2013.04.002. Epub 2013 Apr 10. Mol Genet Metab. 2013. PMID: 23665161 Free PMC article. Review.
-
Porcine N-acetylgalactosamine 6-sulfatase (GALNS) cDNA sequence and expression in developing teeth.Connect Tissue Res. 2002;43(2-3):167-75. doi: 10.1080/03008200290001131. Connect Tissue Res. 2002. PMID: 12489154
Cited by
-
Lentiviral Vector-Mediated Ex Vivo Hematopoietic Stem Cell Gene Therapy for Mucopolysaccharidosis IVA Murine Model.Hum Gene Ther. 2024 Nov;35(21-22):917-937. doi: 10.1089/hum.2024.094. Epub 2024 Oct 24. Hum Gene Ther. 2024. PMID: 39446675
-
CRISPR/nCas9-Edited CD34+ Cells Rescue Mucopolysaccharidosis IVA Fibroblasts Phenotype.Int J Mol Sci. 2025 May 2;26(9):4334. doi: 10.3390/ijms26094334. Int J Mol Sci. 2025. PMID: 40362571 Free PMC article.
-
CRISPR/Cas-Based Ex Vivo Gene Therapy and Lysosomal Storage Disorders: A Perspective Beyond Cas9.Cells. 2025 Jul 25;14(15):1147. doi: 10.3390/cells14151147. Cells. 2025. PMID: 40801580 Free PMC article. Review.
-
Potential Targeting Mechanisms for Bone-Directed Therapies.Int J Mol Sci. 2024 Jul 30;25(15):8339. doi: 10.3390/ijms25158339. Int J Mol Sci. 2024. PMID: 39125906 Free PMC article. Review.
-
In vivo direct lentiviral gene therapy improves disease pathology in a mucopolysaccharidosis IVA murine model.Mol Ther Methods Clin Dev. 2025 Jun 16;33(3):101514. doi: 10.1016/j.omtm.2025.101514. eCollection 2025 Sep 11. Mol Ther Methods Clin Dev. 2025. PMID: 40677837 Free PMC article.
References
-
- Selvam P., Jain A., Abbott J., Ahuja A.S., Cheema A., Bruno K.A., Atwal H., Forghani I., Caulfield T., Atwal P.S. Molecular Modeling and Phenotypic Description of a Patient with a Novel Exonic Deletion of GALNS with Resultant Morquio Syndrome with Two Successful Pregnancies. Mol. Syndromol. 2022;13:282–289. doi: 10.1159/000519326. - DOI - PMC - PubMed
-
- Frigeni M., Rodriguez-Buritica D.F., Saavedra H., Gunther K.A., Hillman P.R., Balaguru D., Northrup H. The youngest pair of siblings with Mucopolysaccharidosis type IVA to receive enzyme replacement therapy to date: A case report. Am. J. Med. Genet. Part A. 2021;185:3510–3516. doi: 10.1002/ajmg.a.62469. - DOI - PubMed
-
- Quijada-Fraile P., Canales E.A., Martín-Hernández E., Ballesta-Martínez M.J., Guillén-Navarro E., Pintos-Morell G., Moltó-Abad M., Moreno-Martínez D., Morillo S.G., Blasco-Alonso J., et al. Clinical features and health-related quality of life in adult patients with mucopolysaccharidosis IVA: The Spanish experience. Orphanet J. Rare Dis. 2021;16:464. doi: 10.1186/s13023-021-02074-y. - DOI - PMC - PubMed
-
- Akyol M.U., MPS Consensus Programme Steering Committee. Alden T.D., Amartino H., Ashworth J., Belani K., Berger K.I., Borgo A., Braunlin E., Eto Y., et al. Recommendations for the management of MPS IVA: Systematic evidence- and consensus-based guidance. Orphanet J. Rare Dis. 2019;14:137. doi: 10.1186/s13023-019-1074-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

