Exploring the mRNA and Plasma Protein Levels of BDNF, NT4, SIRT1, HSP27, and HSP70 in Multiple Sclerosis Patients and Healthy Controls
- PMID: 38003363
- PMCID: PMC10671202
- DOI: 10.3390/ijms242216176
Exploring the mRNA and Plasma Protein Levels of BDNF, NT4, SIRT1, HSP27, and HSP70 in Multiple Sclerosis Patients and Healthy Controls
Abstract
Multiple sclerosis (MS) is a chronic, autoimmune neurodegenerative disease affecting the central nervous system. It is a major cause of non-traumatic neurological disability among young adults in North America and Europe. This study focuses on neuroprotective genes (BDNF, NT4/5, SIRT1, HSP70, and HSP27). Gene expression and protein levels of these markers were compared between MS patients and healthy controls. Blood samples were collected from 42 patients with multiple sclerosis (MS) and 48 control subjects without MS. Quantitative real-time PCR was performed to measure the expression of specific genes. The samples were analyzed in duplicate, and the abundance of mRNA was quantified using the 2-ΔCt method. ELISA assay was used to measure the concentration of specific proteins in the plasma samples. The results show that a 3.5-fold decrease in the gene expression of BDNF corresponds to a 1.5-fold downregulation in the associated plasma protein concentration (p < 0.001). Similar trends were observed with NT-4 (five-fold decrease, slight elevation in protein), SIRT1 (two-fold decrease, two-fold protein decrease), HSP70 (four-fold increase, nearly two-fold protein increase), and HSP27 (four-fold increase, two-fold protein increase) (p < 0.001). This study reveals strong correlations between gene expression and protein concentration in MS patients, emphasizing the relevance of these neuroprotective markers in the disease.
Keywords: BDNF; ELISA; HSP27; HSP70; NT-4; SIRT1; correlation; gene expression; multiple sclerosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

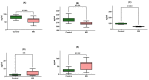

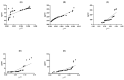
Similar articles
-
Association of miRNA and mRNA Levels of the Clinical Onset of Multiple Sclerosis Patients.Biology (Basel). 2021 Jun 20;10(6):554. doi: 10.3390/biology10060554. Biology (Basel). 2021. PMID: 34202956 Free PMC article.
-
Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans.Am J Physiol Regul Integr Comp Physiol. 2007 Aug;293(2):R844-53. doi: 10.1152/ajpregu.00677.2006. Epub 2007 May 23. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 17522120
-
Heat shock protein 70-hom gene polymorphism and protein expression in multiple sclerosis.J Neuroimmunol. 2016 Sep 15;298:189-93. doi: 10.1016/j.jneuroim.2016.07.011. Epub 2016 Jul 15. J Neuroimmunol. 2016. PMID: 27609295
-
The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system.Int J Hyperthermia. 2005 Aug;21(5):379-92. doi: 10.1080/02656730500069955. Int J Hyperthermia. 2005. PMID: 16048836 Review.
-
Heat shock protein 70: roles in multiple sclerosis.Mol Med. 2012 Sep 7;18(1):1018-28. doi: 10.2119/molmed.2012.00119. Mol Med. 2012. PMID: 22669475 Free PMC article. Review.
Cited by
-
Proteomic analysis reveals candidate molecules to mediate cortical pathology and identify possible biomarkers in an animal model of multiple sclerosis.Front Immunol. 2025 Feb 13;16:1505459. doi: 10.3389/fimmu.2025.1505459. eCollection 2025. Front Immunol. 2025. PMID: 40018028 Free PMC article.
-
Are Sirtuins 1 and 2 Relevant Players in Relapsing-Remitting Multiple Sclerosis?Biomedicines. 2024 Sep 5;12(9):2027. doi: 10.3390/biomedicines12092027. Biomedicines. 2024. PMID: 39335541 Free PMC article.
-
Peripheral Brain-Derived Neurotrophic Factor (BDNF) and Its Regulatory miRNAs as Biological Correlates of Impulsivity in Young Adults.Metabolites. 2024 Sep 30;14(10):529. doi: 10.3390/metabo14100529. Metabolites. 2024. PMID: 39452910 Free PMC article.
-
Exploring the Gene Expression and Plasma Protein Levels of HSP90, HSP60, and GDNF in Multiple Sclerosis Patients and Healthy Controls.Curr Issues Mol Biol. 2024 Oct 19;46(10):11668-11680. doi: 10.3390/cimb46100693. Curr Issues Mol Biol. 2024. PMID: 39451573 Free PMC article.
-
Brain-Derived Neurotrophic Factor in Multiple Sclerosis Disability: A Prospective Study.Brain Sci. 2024 Feb 29;14(3):243. doi: 10.3390/brainsci14030243. Brain Sci. 2024. PMID: 38539631 Free PMC article.
References
-
- Dutta R., Trapp B.D. Relapsing and progressive forms of multiple sclerosis: Insights from pathology. [(accessed on 6 November 2023)];Curr. Opin. Neurol. 2014 27:271–278. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24722325. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

