Dual RNA-Seq Analysis Pinpoints a Balanced Regulation between Symbiosis and Immunity in Medicago truncatula- Sinorhizobium meliloti Symbiotic Nodules
- PMID: 38003367
- PMCID: PMC10671737
- DOI: 10.3390/ijms242216178
Dual RNA-Seq Analysis Pinpoints a Balanced Regulation between Symbiosis and Immunity in Medicago truncatula- Sinorhizobium meliloti Symbiotic Nodules
Abstract
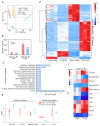
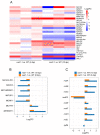
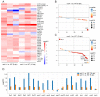
Legume-rhizobial symbiosis initiates the formation of root nodules, within which rhizobia reside and differentiate into bacteroids to convert nitrogen into ammonium, facilitating plant growth. This process raises a fundamental question: how is plant immunity modulated within nodules when exposed to a substantial number of foreign bacteria? In Medicago truncatula, a mutation in the NAD1 (Nodules with Activated Defense 1) gene exclusively results in the formation of necrotic nodules combined with activated immunity, underscoring the critical role of NAD1 in suppressing immunity within nodules. In this study, we employed a dual RNA-seq transcriptomic technology to comprehensively analyze gene expression from both hosts and symbionts in the nad1-1 mutant nodules at different developmental stages (6 dpi and 10 dpi). We identified 89 differentially expressed genes (DEGs) related to symbiotic nitrogen fixation and 89 DEGs from M. truncatula associated with immunity in the nad1-1 nodules. Concurrently, we identified 27 rhizobial DEGs in the fix and nif genes of Sinorhizobium meliloti. Furthermore, we identified 56 DEGs from S. meliloti that are related to stress responses to ROS and NO. Our analyses of nitrogen fixation-defective plant nad1-1 mutants with overactivated defenses suggest that the host employs plant immunity to regulate the substantial bacterial colonization in nodules. These findings shed light on the role of NAD1 in inhibiting the plant's immune response to maintain numerous rhizobial endosymbiosis in nodules.
Keywords: Medicago truncatula; NAD1; Sinorhizobium meliloti; dual RNA-seq; legume–rhizobial symbiosis; plant defense response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Sinorhizobium medicae WSM419 Genes That Improve Symbiosis between Sinorhizobium meliloti Rm1021 and Medicago truncatula Jemalong A17 and in Other Symbiosis Systems.Appl Environ Microbiol. 2021 Jul 13;87(15):e0300420. doi: 10.1128/AEM.03004-20. Epub 2021 Jul 13. Appl Environ Microbiol. 2021. PMID: 33990306 Free PMC article.
-
Transcriptomic Analysis of Sinorhizobium meliloti and Medicago truncatula Symbiosis Using Nitrogen Fixation-Deficient Nodules.Mol Plant Microbe Interact. 2015 Aug;28(8):856-68. doi: 10.1094/MPMI-12-14-0407-R. Epub 2015 Jul 16. Mol Plant Microbe Interact. 2015. PMID: 25844838
-
NODULES WITH ACTIVATED DEFENSE 1 is required for maintenance of rhizobial endosymbiosis in Medicago truncatula.New Phytol. 2016 Oct;212(1):176-91. doi: 10.1111/nph.14017. Epub 2016 Jun 1. New Phytol. 2016. PMID: 27245091
-
The Multiple Faces of the Medicago-Sinorhizobium Symbiosis.Methods Mol Biol. 2018;1822:241-260. doi: 10.1007/978-1-4939-8633-0_16. Methods Mol Biol. 2018. PMID: 30043308 Review.
-
How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model.Nat Rev Microbiol. 2007 Aug;5(8):619-33. doi: 10.1038/nrmicro1705. Nat Rev Microbiol. 2007. PMID: 17632573 Free PMC article. Review.
Cited by
-
Defense and senescence interplay in legume nodules.Plant Commun. 2024 Apr 8;5(4):100888. doi: 10.1016/j.xplc.2024.100888. Epub 2024 Mar 26. Plant Commun. 2024. PMID: 38532645 Free PMC article. Review.
-
CLE peptide signaling in plant-microbe interactions.Front Plant Sci. 2024 Oct 23;15:1481650. doi: 10.3389/fpls.2024.1481650. eCollection 2024. Front Plant Sci. 2024. PMID: 39507357 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

