Evaluation of the Antimicrobial Activity of a Formulation Containing Ascorbic Acid and Eudragit FS 30D Microparticles for the Controlled Release of a Curcumin-Boric Acid Solid Dispersion in Turkey Poults Infected with Salmonella enteritidis: A Therapeutic Model
- PMID: 38003375
- PMCID: PMC10671343
- DOI: 10.3390/ijms242216186
Evaluation of the Antimicrobial Activity of a Formulation Containing Ascorbic Acid and Eudragit FS 30D Microparticles for the Controlled Release of a Curcumin-Boric Acid Solid Dispersion in Turkey Poults Infected with Salmonella enteritidis: A Therapeutic Model
Abstract
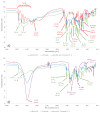
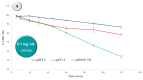
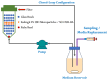
The selection of components within a formulation or for treatment must stop being arbitrary and must be focused on scientific evidence that supports the inclusion of each one. Therefore, the objective of the present study was to obtain a formulation based on ascorbic acid (AA) and Eudragit FS 30D microparticles containing curcumin-boric acid (CUR-BA) considering interaction studies between the active components carried out via Fourier transform infrared spectrometry (FTIR) and differential scanning calorimetry (DSC) to minimize antagonistic effects, and comprehensively and effectively treat turkey poults infected with Salmonella enteritidis (S. enteritidis). The DSC and FTIR studies clearly demonstrated the interactions between AA, BA, and CUR. Consequently, the combination of AA with CUR and/or BA should be avoided, but not CUR and BA. Furthermore, the Eudragit FS 30D microparticles containing CUR-BA (SD CUR-BA MP) showed a limited release of CUR-BA in an acidic medium, but they were released at a pH 6.8-7.0, which reduced the interactions between CUR-BA and AA. Finally, in the S. enteritidis infection model, turkey poults treated with the combination of AA and SD CUR-BA MP presented lower counts of S. enteritidis in cecal tonsils after 10 days of treatment. These results pointed out that the use of an adequate combination of AA and CUR-BA as an integral treatment of S. enteritidis infections could be a viable option to replace the indiscriminate use of antibiotics.
Keywords: Eudragit FS 30D; S. enteritidis; ascorbic acid; boric acid; curcumin; degradation studies; interaction studies; release studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Evaluation of the Dietary Supplementation of a Formulation Containing Ascorbic Acid and a Solid Dispersion of Curcumin with Boric Acid against Salmonella Enteritidis and Necrotic Enteritis in Broiler Chickens.Animals (Basel). 2019 Apr 22;9(4):184. doi: 10.3390/ani9040184. Animals (Basel). 2019. PMID: 31013587 Free PMC article.
-
Comparison of PrestoBlue® and plating method to evaluate antimicrobial activity of ascorbic acid, boric acid and curcumin in an in vitro gastrointestinal model.J Appl Microbiol. 2018 Feb;124(2):423-430. doi: 10.1111/jam.13659. Epub 2018 Jan 8. J Appl Microbiol. 2018. PMID: 29215799
-
Evaluation of a Solid Dispersion of Curcumin With Polyvinylpyrrolidone and Boric Acid Against Salmonella Enteritidis Infection and Intestinal Permeability in Broiler Chickens: A Pilot Study.Front Microbiol. 2018 Jun 15;9:1289. doi: 10.3389/fmicb.2018.01289. eCollection 2018. Front Microbiol. 2018. PMID: 29973919 Free PMC article.
-
Evaluation of Ascorbic Acid or Curcumin Formulated in a Solid Dispersion on Salmonella Enteritidis Infection and Intestinal Integrity in Broiler Chickens.Pathogens. 2019 Nov 10;8(4):229. doi: 10.3390/pathogens8040229. Pathogens. 2019. PMID: 31717681 Free PMC article.
-
Evaluation of the antimicrobial and intestinal integrity properties of boric acid in broiler chickens infected with Salmonella enteritidis: Proof of concept.Res Vet Sci. 2019 Apr;123:7-13. doi: 10.1016/j.rvsc.2018.12.004. Epub 2018 Dec 14. Res Vet Sci. 2019. PMID: 30579139
References
-
- Hamed E.A., Abdelaty M.F., Sorour H.K., Elmasry D., Abdelmagid M.A., Saleh M.A.M., AbdelRahman M.A.A. A pilot study on the effect of thyme microemulsion compared with antibiotic as treatment of Salmonella enteritidis in broiler. Vet. Med. Int. 2022;2022:3647523. doi: 10.1155/2022/3647523. - DOI - PMC - PubMed
-
- Li Y., Kang X., Ed-Dra A., Zhou X., Jia C., Müller A., Liu Y., Kehrenberg C., Yue M. Genome-based assessment of antimicrobial resistance and virulence potential of isolates of non-pullorum/gallinarum Salmonella Serovars recovered from dead poultry in China. Microbiol. Spectr. 2022;10:e00965-22. doi: 10.1128/spectrum.00965-22. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

