Bisphenol AF Induces Prostatic Dorsal Lobe Hyperplasia in Rats through Activation of the NF-κB Signaling Pathway
- PMID: 38003411
- PMCID: PMC10671145
- DOI: 10.3390/ijms242216221
Bisphenol AF Induces Prostatic Dorsal Lobe Hyperplasia in Rats through Activation of the NF-κB Signaling Pathway
Abstract
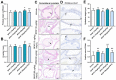
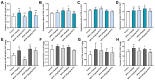
Bisphenol AF (BPAF) represents a common environmental estrogenic compound renowned for its capacity to induce endocrine disruptions. Notably, BPAF exhibits an enhanced binding affinity to estrogen receptors, which may have more potent estrogenic activity compared with its precursor bisphenol A (BPA). Notwithstanding, the existing studies on BPAF-induced prostate toxicity remain limited, with related toxicological research residing in the preliminary stage. Our previous studies have confirmed the role of BPAF in the induction of ventral prostatic hyperplasia, but its role in the dorsal lobe is not clear. In this study, BPAF (10, 90 μg/kg) and the inhibitor of nuclear transcription factor-κB (NF-κB), pyrrolidinedithiocarbamate (PDTC, 100 mg/kg), were administered intragastrically in rats for four weeks. Through comprehensive anatomical and pathological observations, as well as the assessment of PCNA over-expression, we asserted that BPAF at lower doses may foster dorsal prostatic hyperplasia in rats. The results of IHC and ELISA indicated that BPAF induced hyperplastic responses in the dorsal lobe of the prostate by interfering with a series of biomarkers in NF-κB signaling pathways, containing NF-κB p65, COX-2, TNF-α, and EGFR. These findings confirm the toxic effect of BPAF on prostate health and emphasize the potential corresponding mechanisms.
Keywords: bisphenol AF; cyclooxygenase-2; nuclear transcription factor-κB; prostatic hyperplasia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Individual and Combined Effect of Bisphenol A and Bisphenol AF on Prostate Cell Proliferation through NF-κB Signaling Pathway.Int J Mol Sci. 2022 Oct 14;23(20):12283. doi: 10.3390/ijms232012283. Int J Mol Sci. 2022. PMID: 36293141 Free PMC article.
-
The prostaglandin synthases, COX-2 and L-PGDS, mediate prostate hyperplasia induced by low-dose bisphenol A.Sci Rep. 2020 Aug 4;10(1):13108. doi: 10.1038/s41598-020-69809-y. Sci Rep. 2020. PMID: 32753632 Free PMC article.
-
Neurotoxic effects of bisphenol AF on calcium-induced ROS and MAPKs.Neurotox Res. 2013 Apr;23(3):249-59. doi: 10.1007/s12640-012-9353-4. Epub 2012 Sep 21. Neurotox Res. 2013. PMID: 22996013
-
Prenatal exposure to bisphenol AF induced male offspring reproductive dysfunction by triggering testicular innate and adaptive immune responses.Ecotoxicol Environ Saf. 2023 Jul 1;259:115030. doi: 10.1016/j.ecoenv.2023.115030. Epub 2023 May 20. Ecotoxicol Environ Saf. 2023. PMID: 37216864
-
Bisphenol AF promotes estrogen receptor-positive breast cancer cell proliferation through amphiregulin-mediated crosstalk with receptor tyrosine kinase signaling.PLoS One. 2019 May 6;14(5):e0216469. doi: 10.1371/journal.pone.0216469. eCollection 2019. PLoS One. 2019. PMID: 31059536 Free PMC article.
References
-
- Zoeller R.T., Brown T.R., Doan L.L., Gore A.C., Skakkebaek N.E., Soto A.M., Woodruff T.J., Vom Saal F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

