Modulating Stress Proteins in Response to Therapeutic Interventions for Parkinson's Disease
- PMID: 38003423
- PMCID: PMC10671288
- DOI: 10.3390/ijms242216233
Modulating Stress Proteins in Response to Therapeutic Interventions for Parkinson's Disease
Abstract
Parkinson's disease (PD) is a neurodegenerative illness characterized by the degeneration of dopaminergic neurons in the substantia nigra, resulting in motor symptoms and without debilitating motors. A hallmark of this condition is the accumulation of misfolded proteins, a phenomenon that drives disease progression. In this regard, heat shock proteins (HSPs) play a central role in the cellular response to stress, shielding cells from damage induced by protein aggregates and oxidative stress. As a result, researchers have become increasingly interested in modulating these proteins through pharmacological and non-pharmacological therapeutic interventions. This review aims to provide an overview of the preclinical experiments performed over the last decade in this research field. Specifically, it focuses on preclinical studies that center on the modulation of stress proteins for the treatment potential of PD. The findings display promise in targeting HSPs to ameliorate PD outcomes. Despite the complexity of HSPs and their co-chaperones, proteins such as HSP70, HSP27, HSP90, and glucose-regulated protein-78 (GRP78) may be efficacious in slowing or preventing disease progression. Nevertheless, clinical validation is essential to confirm the safety and effectiveness of these preclinical approaches.
Keywords: Parkinson’s disease; heat shock proteins; neuroregeneration; nonpharmacological interventions; pharmacological modulation; protein misfolding; stress protein modulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
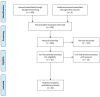
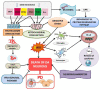

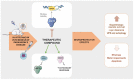

References
-
- Schneider S.A., Obeso J.A. Clinical and pathological features of parkinson’s disease. Behav. Neurobiol. Huntington’s Dis. Park. Dis. 2015;22:205–220. - PubMed
-
- Kudryavtseva A.V., Krasnov G.S., Dmitriev A.A., Alekseev B.Y., Kardymon O.L., Sadritdinova A.F., Fedorova M.S., Pokrovsky A.V., Melnikova N.V., Kaprin A.D., et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7:44879–44905. doi: 10.18632/oncotarget.9821. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

