Tumor Regression upon Intratumoral and Subcutaneous Dosing of the STING Agonist ALG-031048 in Mouse Efficacy Models
- PMID: 38003463
- PMCID: PMC10671074
- DOI: 10.3390/ijms242216274
Tumor Regression upon Intratumoral and Subcutaneous Dosing of the STING Agonist ALG-031048 in Mouse Efficacy Models
Abstract
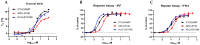
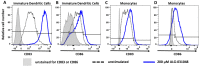
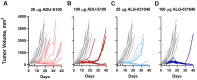
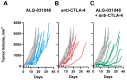
Stimulator of interferon genes (STING) agonists have shown potent anti-tumor efficacy in various mouse tumor models and have the potential to overcome resistance to immune checkpoint inhibitors (ICI) by linking the innate and acquired immune systems. First-generation STING agonists are administered intratumorally; however, a systemic delivery route would greatly expand the clinical use of STING agonists. Biochemical and cell-based experiments, as well as syngeneic mouse efficacy models, were used to demonstrate the anti-tumoral activity of ALG-031048, a novel STING agonist. In vitro, ALG-031048 is highly stable in plasma and liver microsomes and is resistant to degradation via phosphodiesterases. The high stability in biological matrices translated to good cellular potency in a HEK 293 STING R232 reporter assay, efficient activation and maturation of primary human dendritic cells and monocytes, as well as long-lasting, antigen-specific anti-tumor activity in up to 90% of animals in the CT26 mouse colon carcinoma model. Significant reductions in tumor growth were observed in two syngeneic mouse tumor models following subcutaneous administration. Combinations of ALG-031048 and ICIs further enhanced the in vivo anti-tumor activity. This initial demonstration of anti-tumor activity after systemic administration of ALG-031048 warrants further investigation, while the combination of systemically administered ALG-031048 with ICIs offers an attractive approach to overcome key limitations of ICIs in the clinic.
Keywords: STING agonist; immune checkpoint inhibitor; immuno-oncology; syngeneic mouse model.
Conflict of interest statement
The authors declare the following interest that may be considered as potential competing interest: At the time of the study, all authors with the exception of F.G. were employees of Aligos Therapeutics, Inc., while F.G. was an employee of Aligos Belgium BV. Patent applications for ALG-031048 are pending. The authors declare that the study was funded in its entirety by Aligos Therapeutics. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Figures


Similar articles
-
Overcome the challenge for intratumoral injection of STING agonist for pancreatic cancer by systemic administration.J Hematol Oncol. 2024 Aug 7;17(1):62. doi: 10.1186/s13045-024-01576-z. J Hematol Oncol. 2024. PMID: 39113096 Free PMC article.
-
STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer.J Immunother Cancer. 2021 Jun;9(6):e002195. doi: 10.1136/jitc-2020-002195. J Immunother Cancer. 2021. PMID: 34145029 Free PMC article.
-
Structure-Activity relationship study of benzothiophene oxobutanoic acid analogues leading to novel stimulator of interferon gene (STING) agonists.Eur J Med Chem. 2022 Nov 5;241:114627. doi: 10.1016/j.ejmech.2022.114627. Epub 2022 Aug 7. Eur J Med Chem. 2022. PMID: 35963129
-
Challenges and Opportunities in the Clinical Development of STING Agonists for Cancer Immunotherapy.J Clin Med. 2020 Oct 16;9(10):3323. doi: 10.3390/jcm9103323. J Clin Med. 2020. PMID: 33081170 Free PMC article. Review.
-
The Development of STING Agonists and Emerging Results as a Cancer Immunotherapy.Curr Oncol Rep. 2023 Mar;25(3):189-199. doi: 10.1007/s11912-023-01361-0. Epub 2023 Jan 27. Curr Oncol Rep. 2023. PMID: 36705879 Free PMC article. Review.
Cited by
-
Targeting STING signaling for the optimal cancer immunotherapy.Front Immunol. 2024 Oct 9;15:1482738. doi: 10.3389/fimmu.2024.1482738. eCollection 2024. Front Immunol. 2024. PMID: 39450170 Free PMC article. Review.
-
Demystifying the cGAS-STING pathway: precision regulation in the tumor immune microenvironment.Mol Cancer. 2025 Jun 12;24(1):178. doi: 10.1186/s12943-025-02380-0. Mol Cancer. 2025. PMID: 40506729 Free PMC article. Review.
-
The activation of cGAS-STING pathway offers novel therapeutic opportunities in cancers.Front Immunol. 2025 Jun 9;16:1579832. doi: 10.3389/fimmu.2025.1579832. eCollection 2025. Front Immunol. 2025. PMID: 40552295 Free PMC article. Review.
-
Beyond Adaptive Immunity: Trained Innate Immune Responses as a Novel Frontier in Hepatocellular Carcinoma Therapy.Cancers (Basel). 2025 Apr 7;17(7):1250. doi: 10.3390/cancers17071250. Cancers (Basel). 2025. PMID: 40227782 Free PMC article. Review.
-
Liver Transplantation for Non-hepatocellular Carcinoma: The Role of Immune Checkpoint Inhibitors.J Clin Exp Hepatol. 2025 Sep-Oct;15(5):102558. doi: 10.1016/j.jceh.2025.102558. Epub 2025 Mar 27. J Clin Exp Hepatol. 2025. PMID: 40303874 Review.
References
-
- Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., Li X.-D., Mauceri H., Beckett M., Darga T., et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. - DOI - PMC - PubMed
-
- Woo S.-R., Fuertes M.B., Corrales L., Spranger S., Furdyna M.J., Leung M.Y.K., Duggan R., Wang Y., Barber G.N., Fitzgerald K.A., et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

