Graft-Host Interaction and Its Effect on Wound Repair Using Mouse Models
- PMID: 38003467
- PMCID: PMC10671506
- DOI: 10.3390/ijms242216277
Graft-Host Interaction and Its Effect on Wound Repair Using Mouse Models
Abstract
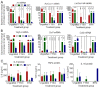
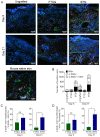
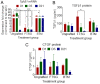
Autologous skin grafting has been commonly used in clinics for decades to close large wounds, yet the cellular and molecular interactions between the wound bed and the graft that mediates the wound repair are not fully understood. The aim of this study was to better understand the molecular changes in the wound triggered by autologous and synthetic grafting. Defining the wound changes at the molecular level during grafting sets the basis to test other engineered skin grafts by design. In this study, a full-thickness skin graft (SKH-1 hairless) mouse model was established. An autologous full-thickness skin graft (FTSG) or an acellular fully synthetic Biodegradable Temporising Matrix (BTM) was grafted. The wound bed/grafts were analysed at histological, RNA, and protein levels during the inflammation (day 1), proliferation (day 5), and remodelling (day 21) phases of wound repair. The results showed that in this mouse model, similar to others, inflammatory marker levels, including Il-6, Cxcl-1, and Cxcl-5/6, were raised within a day post-wounding. Autologous grafting reduced the expression of these inflammatory markers. This was different from the wounds grafted with synthetic dermal grafts, in which Cxcl-1 and Cxcl-5/6 remained significantly high up to 21 days post-grafting. Autologous skin grafting reduced wound contraction compared to wounds that were left to spontaneously repair. Synthetic grafts contracted significantly more than FTSG by day 21. The observed wound contraction in synthetic grafts was most likely mediated at least partly by myofibroblasts. It is possible that high TGF-β1 levels in days 1-21 were the driving force behind myofibroblast abundance in synthetic grafts, although no evidence of TGF-β1-mediated Connective Tissue Growth Factor (CTGF) upregulation was observed.
Keywords: IL-6; TGF-β1; myofibroblast; skin grafting; wound repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A Comparative Study of Engineered Dermal Templates for Skin Wound Repair in a Mouse Model.Int J Mol Sci. 2020 Jun 25;21(12):4508. doi: 10.3390/ijms21124508. Int J Mol Sci. 2020. PMID: 32630398 Free PMC article.
-
Split-Thickness Skin Grafts.2025 Feb 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Feb 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31855388 Free Books & Documents.
-
[Clinical effect of bi-layered artificial dermis and autologous skin graft in repairing bone and/or tendon exposed wounds].Zhonghua Shao Shang Za Zhi. 2020 Mar 20;36(3):179-186. doi: 10.3760/cma.j.cn501120-20191119-00437. Zhonghua Shao Shang Za Zhi. 2020. PMID: 32241043 Chinese.
-
Understanding the mechanisms of spontaneous and skin-grafted wound repair: the path to engineered skin grafts.J Wound Care. 2023 Jan 2;32(1):55-62. doi: 10.12968/jowc.2023.32.1.55. J Wound Care. 2023. PMID: 36630112 Review.
-
The use of NovoSorb biodegradable temporising matrix in wound management: a literature review and case series.J Wound Care. 2023 Aug 2;32(8):470-478. doi: 10.12968/jowc.2023.32.8.470. J Wound Care. 2023. PMID: 37572341 Review.
References
-
- Enoch S., Leaper D.J. Basic science of wound healing. Surgery. 2008;26:31–37.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

