Epidermal Growth Factor Receptors in Vascular Endothelial Cells Contribute to Functional Hyperemia in the Brain
- PMID: 38003472
- PMCID: PMC10671586
- DOI: 10.3390/ijms242216284
Epidermal Growth Factor Receptors in Vascular Endothelial Cells Contribute to Functional Hyperemia in the Brain
Abstract
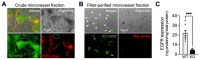
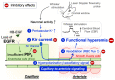
Functional hyperemia-activity-dependent increases in local blood perfusion-underlies the on-demand delivery of blood to regions of enhanced neuronal activity, a process that is crucial for brain health. Importantly, functional hyperemia deficits have been linked to multiple dementia risk factors, including aging, chronic hypertension, and cerebral small vessel disease (cSVD). We previously reported crippled functional hyperemia in a mouse model of genetic cSVD that was likely caused by depletion of phosphatidylinositol 4,5-bisphosphate (PIP2) in capillary endothelial cells (EC) downstream of impaired epidermal growth factor receptor (EGFR) signaling. Here, using EC-specific EGFR-knockout (KO) mice, we directly examined the role of endothelial EGFR signaling in functional hyperemia, assessed by measuring increases in cerebral blood flow in response to contralateral whisker stimulation using laser Doppler flowmetry. Molecular characterizations showed that EGFR expression was dramatically decreased in freshly isolated capillaries from EC-EGFR-KO mice, as expected. Notably, whisker stimulation-induced functional hyperemia was significantly impaired in these mice, an effect that was rescued by administration of PIP2, but not by the EGFR ligand, HB-EGF. These data suggest that the deletion of the EGFR specifically in ECs attenuates functional hyperemia, likely via depleting PIP2 and subsequently incapacitating Kir2.1 channel functionality in capillary ECs. Thus, our study underscores the role of endothelial EGFR signaling in functional hyperemia of the brain.
Keywords: cerebral small vessel diseases (cSVD); epidermal growth factor receptor (EGFR); functional hyperemia; phosphatidylinositol 4,5-bisphosphate (PIP2); vascular endothelial cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
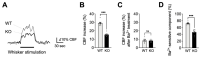

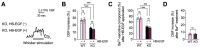
Update of
-
Epidermal growth factor receptors in vascular endothelial cells contribute to functional hyperemia in the brain.bioRxiv [Preprint]. 2023 Sep 17:2023.09.15.557981. doi: 10.1101/2023.09.15.557981. bioRxiv. 2023. Update in: Int J Mol Sci. 2023 Nov 14;24(22):16284. doi: 10.3390/ijms242216284. PMID: 37745396 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

