Gut Microbiome-Estrobolome Profile in Reproductive-Age Women with Endometriosis
- PMID: 38003489
- PMCID: PMC10671785
- DOI: 10.3390/ijms242216301
Gut Microbiome-Estrobolome Profile in Reproductive-Age Women with Endometriosis
Abstract
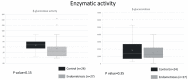
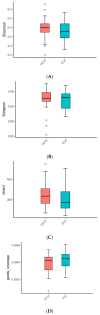
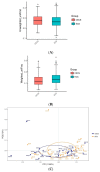
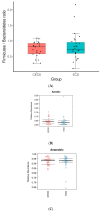
Microbiota is associated with our bodily functions and microenvironment. A healthy, balanced gut microbiome not only helps maintain mucosal integrity, prevents translocation of bacterial content, and contributes to immune status, but also associates with estrogen metabolism. Gut dysbiosis and estrobolome dysfunction have hence been linked to certain estrogen-dependent diseases, including endometriosis. While prior studies on microbiomes and endometriosis have shown conflicting results, most of the observed microbial differences are seen in the genital tract. This case-control study of reproductive-age women utilizes their fecal and urine samples for enzymatic, microbial, and metabolic studies to explore if patients with endometriosis have distinguishable gut microbiota or altered estrogen metabolism. While gut β-glucuronidase activities, microbial diversity, and abundance did not vary significantly between patients with or without endometriosis, fecal samples of patients with endometriosis were more enriched by the Erysipelotrichia class and had higher folds of four estrogen/estrogen metabolites. Further studies are needed to elucidate what these results imply and whether there indeed is an association or causation between gut microbiota and endometriosis.
Keywords: 16S ribosomal-RNA gene; dysbiosis; endometriosis; estrogen metabolites; gut microbiota; β-glucuronidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

