Buckwheat (Fagopyrum esculentum) Hulls Are a Rich Source of Fermentable Dietary Fibre and Bioactive Phytochemicals
- PMID: 38003497
- PMCID: PMC10671810
- DOI: 10.3390/ijms242216310
Buckwheat (Fagopyrum esculentum) Hulls Are a Rich Source of Fermentable Dietary Fibre and Bioactive Phytochemicals
Abstract
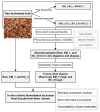
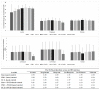
Pseudo-cereals such as buckwheat (Fagopyrum esculentum) are valid candidates to promote diet biodiversity and nutrition security in an era of global climate change. Buckwheat hulls (BHs) are currently an unexplored source of dietary fibre and bioactive phytochemicals. This study assessed the effects of several bioprocessing treatments (using enzymes, yeast, and combinations of both) on BHs' nutrient and phytochemical content, their digestion and metabolism in vitro (using a gastrointestinal digestion model and mixed microbiota from human faeces). The metabolites were measured using targeted LC-MS/MS and GC analysis and 16S rRNA gene sequencing was used to detect the impact on microbiota composition. BHs are rich in insoluble fibre (31.09 ± 0.22% as non-starch polysaccharides), protocatechuic acid (390.71 ± 31.72 mg/kg), and syringaresinol (125.60 ± 6.76 mg/kg). The bioprocessing treatments significantly increased the extractability of gallic acid, vanillic acid, p-hydroxybenzoic acid, syringic acid, vanillin, syringaldehyde, p-coumaric acid, ferulic acid, caffeic acid, and syringaresinol in the alkaline-labile bound form, suggesting the bioaccessibility of these phytochemicals to the colon. Furthermore, one of the treatments, EC_2 treatment, increased significantly the in vitro upper gastrointestinal release of bioactive phytochemicals, especially for protocatechuic acid (p < 0.01). The BH fibre was fermentable, promoting the formation mainly of propionate and, to a lesser extent, butyrate formation. The EM_1 and EC_2 treatments effectively increased the content of insoluble fibre but had no effect on dietary fibre fermentation (p > 0.05). These findings promote the use of buckwheat hulls as a source of dietary fibre and phytochemicals to help meet dietary recommendations and needs.
Keywords: buckwheat (Fagopyrum esculentum) hulls; dietary fibre; enzyme bioprocessing; gut microbiota; in vitro digestion; in vitro fermentation; microbial metabolites; phytochemicals; short chain fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The Content of Dietary Fibre and Polyphenols in Morphological Parts of Buckwheat (Fagopyrum tataricum).Plant Foods Hum Nutr. 2018 Mar;73(1):82-88. doi: 10.1007/s11130-018-0659-0. Plant Foods Hum Nutr. 2018. PMID: 29435700 Free PMC article.
-
Liquid chromatography-mass spectrometry-based metabolomics analysis of flavonoids and anthraquinones in Fagopyrum tataricum L. Gaertn. (tartary buckwheat) seeds to trace morphological variations.Food Chem. 2020 Nov 30;331:127354. doi: 10.1016/j.foodchem.2020.127354. Epub 2020 Jun 16. Food Chem. 2020. PMID: 32569973
-
Habitual consumption of high-fibre bread fortified with bean hulls increased plasma indole-3-propionic concentration and decreased putrescine and deoxycholic acid faecal concentrations in healthy volunteers.Br J Nutr. 2023 Nov 14;130(9):1521-1536. doi: 10.1017/S0007114523000491. Epub 2023 Feb 27. Br J Nutr. 2023. PMID: 36847278 Free PMC article. Clinical Trial.
-
Buckwheat in Tissue Culture Research: Current Status and Future Perspectives.Int J Mol Sci. 2022 Feb 18;23(4):2298. doi: 10.3390/ijms23042298. Int J Mol Sci. 2022. PMID: 35216414 Free PMC article. Review.
-
Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses.Carbohydr Polym. 2020 Nov 15;248:116819. doi: 10.1016/j.carbpol.2020.116819. Epub 2020 Jul 28. Carbohydr Polym. 2020. PMID: 32919544 Review.
References
-
- Dwivedi S., Sahrawat K., Upadhyaya H., Ortiz R. Food, nutrition and agrobiodiversity under global climate change. Adv. Agron. 2013;120:1–128.
-
- Mijatović D., Van Oudenhoven F., Eyzaguirre P., Hodgkin T. The role of agricultural biodiversity in strengthening resilience to climate change: Towards an analytical framework. Int. J. Agric. Sustain. 2013;11:95–107. doi: 10.1080/14735903.2012.691221. - DOI
-
- Neacsu M., Duncan S. Exploiting Underutilised crops. Food Sci. Technol. 2023;37:36–39.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

