The Controversial Effect of Antibiotics on Methicillin-Sensitive S. aureus: A Comparative In Vitro Study
- PMID: 38003500
- PMCID: PMC10671744
- DOI: 10.3390/ijms242216308
The Controversial Effect of Antibiotics on Methicillin-Sensitive S. aureus: A Comparative In Vitro Study
Abstract
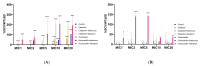
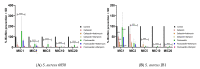
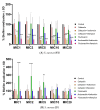
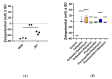
Methicillin-sensitive Staphylococcus (S.) aureus (MSSA) bacteremia remains a global challenge, despite the availability of antibiotics. Primary treatments include β-lactam agents such as cefazolin and flucloxacillin. Ongoing discussions have focused on the potential synergistic effects of combining these agents with rifampicin or fosfomycin to combat infections associated with biofilm formation. Managing staphylococcal infections is challenging due to antibacterial resistance, biofilms, and S. aureus's ability to invade and replicate within host cells. Intracellular invasion shields the bacteria from antibacterial agents and the immune system, often leading to incomplete bacterial clearance and chronic infections. Additionally, S. aureus can assume a dormant phenotype, known as the small colony variant (SCV), further complicating eradication and promoting persistence. This study investigated the impact of antibiotic combinations on the persistence of S. aureus 6850 and its stable small colony variant (SCV strain JB1) focusing on intracellular survival and biofilm formation. The results from the wild-type strain 6850 demonstrate that β-lactams combined with RIF effectively eliminated biofilms and intracellular bacteria but tend to select for SCVs in planktonic culture and host cells. Higher antibiotic concentrations were associated with an increase in the zeta potential of S. aureus, suggesting reduced membrane permeability to antimicrobials. When using the stable SCV mutant strain JB1, antibiotic combinations with rifampicin successfully cleared planktonic bacteria and biofilms but failed to eradicate intracellular bacteria. Given these findings, it is reasonable to report that β-lactams combined with rifampicin represent the optimal treatment for MSSA bacteremia. However, caution is warranted when employing this treatment over an extended period, as it may elevate the risk of selecting for small colony variants (SCVs) and, consequently, promoting bacterial persistence.
Keywords: Staphylococcus aureus; bacteremia; bacterial persistence; cefazolin; flucloxacillin; fosfomycin; rifampicin; small colony variants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Van der Vaart T.W., Prins J.M., Soetekouw R., van Twillert G., Veenstra J., Herpers B.L., Rozemeijer W., Jansen R.R., Bonten M.J.M., van der Meer J.T.M. All-Cause and Infection-Related Mortality in Staphylococcus aureus Bacteremia, a Multicenter Prospective Cohort Study. Open Forum Infect. Dis. 2022;9:ofac653. doi: 10.1093/ofid/ofac653. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

