PROTAC-Based Protein Degradation as a Promising Strategy for Targeted Therapy in Sarcomas
- PMID: 38003535
- PMCID: PMC10671294
- DOI: 10.3390/ijms242216346
PROTAC-Based Protein Degradation as a Promising Strategy for Targeted Therapy in Sarcomas
Abstract
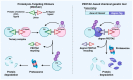
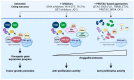
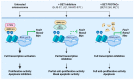
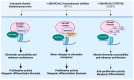
Sarcomas are heterogeneous bone and soft tissue cancers representing the second most common tumor type in children and adolescents. Histology and genetic profiling discovered more than 100 subtypes, which are characterized by peculiar molecular vulnerabilities. However, limited therapeutic options exist beyond standard therapy and clinical benefits from targeted therapies were observed only in a minority of patients with sarcomas. The rarity of these tumors, paucity of actionable mutations, and limitations in the chemical composition of current targeted therapies hindered the use of these approaches in sarcomas. Targeted protein degradation (TPD) is an innovative pharmacological modality to directly alter protein abundance with promising clinical potential in cancer, even for undruggable proteins. TPD is based on the use of small molecules called degraders or proteolysis-targeting chimeras (PROTACs), which trigger ubiquitin-dependent degradation of protein of interest. In this review, we will discuss major features of PROTAC and PROTAC-derived genetic systems for target validation and cancer treatment and focus on the potential of these approaches to overcome major issues connected to targeted therapies in sarcomas, including drug resistance, target specificity, and undruggable targets. A deeper understanding of these strategies might provide new fuel to drive molecular and personalized medicine to sarcomas.
Keywords: BET proteins; BRD9; PROTAC; SMARCA4; degradation tag; fusion genes; sarcomas; targeted therapy; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript or in the decision to publish the manuscript.
Figures
Similar articles
-
Novel strategies and promising opportunities for targeted protein degradation: An innovative therapeutic approach to overcome cancer resistance.Pharmacol Ther. 2023 Apr;244:108371. doi: 10.1016/j.pharmthera.2023.108371. Epub 2023 Mar 5. Pharmacol Ther. 2023. PMID: 36871783 Review.
-
PROTAC 2.0: Expanding the frontiers of targeted protein degradation.Drug Discov Today. 2025 Jun;30(6):104376. doi: 10.1016/j.drudis.2025.104376. Epub 2025 May 8. Drug Discov Today. 2025. PMID: 40348076 Review.
-
Strategies for Precise Modulation of Protein Degradation.Acc Chem Res. 2025 Apr 15;58(8):1236-1248. doi: 10.1021/acs.accounts.5c00003. Epub 2025 Mar 25. Acc Chem Res. 2025. PMID: 40132213 Review.
-
Proteolysis-targeting chimeras (PROTACs) in cancer therapy.Mol Cancer. 2022 Apr 11;21(1):99. doi: 10.1186/s12943-021-01434-3. Mol Cancer. 2022. PMID: 35410300 Free PMC article. Review.
-
[Induced degradation of proteins by PROTACs and other strategies: towards promising drugs].Biol Aujourdhui. 2021;215(1-2):25-43. doi: 10.1051/jbio/2021007. Epub 2021 Aug 16. Biol Aujourdhui. 2021. PMID: 34397373 French.
Cited by
-
Targeting SWI/SNF Complexes in Cancer: Pharmacological Approaches and Implications.Epigenomes. 2024 Feb 4;8(1):7. doi: 10.3390/epigenomes8010007. Epigenomes. 2024. PMID: 38390898 Free PMC article. Review.
-
Protacs in cancer therapy: mechanisms, design, clinical trials, and future directions.Drug Deliv Transl Res. 2025 Jun;15(6):1801-1827. doi: 10.1007/s13346-024-01754-z. Epub 2024 Nov 29. Drug Deliv Transl Res. 2025. PMID: 39614036 Review.
-
Fungi and cancer: unveiling the complex role of fungal infections in tumor biology and therapeutic resistance.Front Cell Infect Microbiol. 2025 Jun 10;15:1596688. doi: 10.3389/fcimb.2025.1596688. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40557321 Free PMC article. Review.
-
Pathology, Diagnosis, and Management of Sarcoma.Int J Mol Sci. 2024 Jun 15;25(12):6609. doi: 10.3390/ijms25126609. Int J Mol Sci. 2024. PMID: 38928315 Free PMC article.
-
Targeted protein degradation: advances in drug discovery and clinical practice.Signal Transduct Target Ther. 2024 Nov 6;9(1):308. doi: 10.1038/s41392-024-02004-x. Signal Transduct Target Ther. 2024. PMID: 39500878 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

