Single-Nuclei Multiome (ATAC + Gene Expression) Sequencing of a Primary Canine Osteosarcoma Elucidates Intra-Tumoral Heterogeneity and Characterizes the Tumor Microenvironment
- PMID: 38003552
- PMCID: PMC10671194
- DOI: 10.3390/ijms242216365
Single-Nuclei Multiome (ATAC + Gene Expression) Sequencing of a Primary Canine Osteosarcoma Elucidates Intra-Tumoral Heterogeneity and Characterizes the Tumor Microenvironment
Abstract
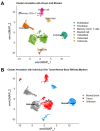
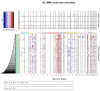
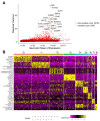
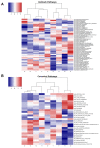
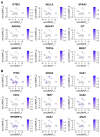
Osteosarcoma (OSA) is a highly aggressive bone tumor primarily affecting pediatric or adolescent humans and large-breed dogs. Canine OSA shares striking similarities with its human counterpart, making it an invaluable translational model for uncovering the disease's complexities and developing novel therapeutic strategies. Tumor heterogeneity, a hallmark of OSA, poses significant challenges to effective treatment due to the evolution of diverse cell populations that influence tumor growth, metastasis, and resistance to therapies. In this study, we apply single-nuclei multiome sequencing, encompassing ATAC (Assay for Transposase-Accessible Chromatin) and GEX (Gene Expression, or RNA) sequencing, to a treatment-naïve primary canine osteosarcoma. This comprehensive approach reveals the complexity of the tumor microenvironment by simultaneously capturing the transcriptomic and epigenomic profiles within the same nucleus. Furthermore, these results are analyzed in conjunction with bulk RNA sequencing and differential analysis of the same tumor and patient-matched normal bone. By delving into the intricacies of OSA at this unprecedented level of detail, we aim to unravel the underlying mechanisms driving intra-tumoral heterogeneity, opening new avenues for therapeutic interventions in both human and canine patients. This study pioneers an approach that is broadly applicable, while demonstrating significant heterogeneity in the context of a single individual's tumor.
Keywords: 10× genomics; canine; dog; heterogeneity; multiome; oncology; osteosarcoma; sequencing; single-nuclei; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
HES1, a target of Notch signaling, is elevated in canine osteosarcoma, but reduced in the most aggressive tumors.BMC Vet Res. 2013 Jul 1;9:130. doi: 10.1186/1746-6148-9-130. BMC Vet Res. 2013. PMID: 23816051 Free PMC article.
-
Transcriptomic Analysis of Canine Osteosarcoma from a Precision Medicine Perspective Reveals Limitations of Differential Gene Expression Studies.Genes (Basel). 2022 Apr 13;13(4):680. doi: 10.3390/genes13040680. Genes (Basel). 2022. PMID: 35456486 Free PMC article.
-
Investigating CXCR4 expression in canine appendicular osteosarcoma.J Vet Intern Med. 2008 May-Jun;22(3):602-8. doi: 10.1111/j.1939-1676.2008.0089.x. Epub 2008 May 2. J Vet Intern Med. 2008. PMID: 18466248
-
Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology.ILAR J. 2014;55(1):69-85. doi: 10.1093/ilar/ilu009. ILAR J. 2014. PMID: 24936031 Review.
-
Comparative Immunology and Immunotherapy of Canine Osteosarcoma.Adv Exp Med Biol. 2020;1258:199-221. doi: 10.1007/978-3-030-43085-6_14. Adv Exp Med Biol. 2020. PMID: 32767244 Review.
Cited by
-
Single-Cell Multi-Omics: Insights into Therapeutic Innovations to Advance Treatment in Cancer.Int J Mol Sci. 2025 Mar 9;26(6):2447. doi: 10.3390/ijms26062447. Int J Mol Sci. 2025. PMID: 40141092 Free PMC article. Review.
-
Review of Molecular Technologies for Investigating Canine Cancer.Animals (Basel). 2024 Feb 29;14(5):769. doi: 10.3390/ani14050769. Animals (Basel). 2024. PMID: 38473154 Free PMC article. Review.
-
Comparative epigenetics of domestic animals: focusing on DNA accessibility and its impact on gene regulation and traits.J Vet Sci. 2025 Jan;26(1):e9. doi: 10.4142/jvs.24259. J Vet Sci. 2025. PMID: 39901471 Free PMC article. Review.
-
Pathology, Diagnosis, and Management of Sarcoma.Int J Mol Sci. 2024 Jun 15;25(12):6609. doi: 10.3390/ijms25126609. Int J Mol Sci. 2024. PMID: 38928315 Free PMC article.
-
The Role of Canine Models of Human Cancer: Overcoming Drug Resistance Through a Transdisciplinary "One Health, One Medicine" Approach.Cancers (Basel). 2025 Jun 17;17(12):2025. doi: 10.3390/cancers17122025. Cancers (Basel). 2025. PMID: 40563676 Free PMC article. Review.
References
-
- Culp W.T.N., Olea-Popelka F., Sefton J., Aldridge C.F., Withrow S.J., Lafferty M.H., Rebhun R.B., Kent M.S., Ehrhart N. Evaluation of Outcome and Prognostic Factors for Dogs Living Greater than One Year after Diagnosis of Osteosarcoma: 90 Cases (1997–2008) J. Am. Vet. Med. Assoc. 2014;245:1141–1146. doi: 10.2460/javma.245.10.1141. - DOI - PMC - PubMed
-
- Mannheimer J.D., Tawa G., Gerhold D., Braisted J., Sayers C.M., McEachron T.A., Meltzer P., Mazcko C., Beck J.A., LeBlanc A.K. Transcriptional Profiling of Canine Osteosarcoma Identifies Prognostic Gene Expression Signatures with Translational Value for Humans. Commun. Biol. 2023;6:856. doi: 10.1038/s42003-023-05208-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

