asb5a/ asb5b Double Knockout Affects Zebrafish Cardiac Contractile Function
- PMID: 38003559
- PMCID: PMC10671462
- DOI: 10.3390/ijms242216364
asb5a/ asb5b Double Knockout Affects Zebrafish Cardiac Contractile Function
Abstract
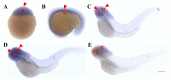
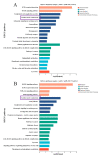
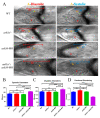
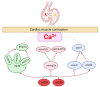
Ankyrin repeat and suppression-of-cytokine-signaling box (Asb) proteins, a subset of ubiquitin ligase E3, include Asb5 with six ankyrin-repeat domains. Zebrafish harbor two asb5 gene isoforms, asb5a and asb5b. Currently, the effects of asb5 gene inactivation on zebrafish embryonic development and heart function are unknown. Using CRISPR/Cas9, we generated asb5a-knockout zebrafish, revealing no abnormal phenotypes at 48 h post-fertilization (hpf). In situ hybridization showed similar asb5a and asb5b expression patterns, indicating the functional redundancy of these isoforms. Morpholino interference was used to target asb5b in wild-type and asb5a-knockout zebrafish. Knocking down asb5b in the wild-type had no phenotypic impact, but simultaneous asb5b knockdown in asb5a-knockout homozygotes led to severe pericardial cavity enlargement and atrial dilation. RNA-seq and cluster analyses identified significantly enriched cardiac muscle contraction genes in the double-knockout at 48 hpf. Moreover, semi-automatic heartbeat analysis demonstrated significant changes in various heart function indicators. STRING database/Cytoscape analyses confirmed that 11 cardiac-contraction-related hub genes exhibited disrupted expression, with three modules containing these genes potentially regulating cardiac contractile function through calcium ion channels. This study reveals functional redundancy in asb5a and asb5b, with simultaneous knockout significantly impacting zebrafish early heart development and contraction, providing key insights into asb5's mechanism.
Keywords: CRISPR/Cas9; RNA-seq; asb5a; asb5b; hub genes; morpholino; semi-automatic heartbeat analysis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures








Similar articles
-
The Interaction Between the asb5a and asb5b Subtypes Jointly Regulates the L-R Asymmetrical Development of the Heart in Zebrafish.Int J Mol Sci. 2025 Mar 19;26(6):2765. doi: 10.3390/ijms26062765. Int J Mol Sci. 2025. PMID: 40141403 Free PMC article.
-
Knockout of tnni1b in zebrafish causes defects in atrioventricular valve development via the inhibition of the myocardial wnt signaling pathway.FASEB J. 2019 Jan;33(1):696-710. doi: 10.1096/fj.201800481RR. Epub 2018 Jul 25. FASEB J. 2019. PMID: 30044923
-
miR-731 modulates the zebrafish heart morphogenesis via targeting Calcineurin/Nfatc3a pathway.Biochim Biophys Acta Gen Subj. 2022 Jun;1866(6):130133. doi: 10.1016/j.bbagen.2022.130133. Epub 2022 Mar 26. Biochim Biophys Acta Gen Subj. 2022. PMID: 35346765
-
Abnormal development of zebrafish after knockout and knockdown of ribosomal protein L10a.Sci Rep. 2019 Dec 2;9(1):18130. doi: 10.1038/s41598-019-54544-w. Sci Rep. 2019. PMID: 31792295 Free PMC article.
-
Heart-specific isoform of tropomyosin4 is essential for heartbeat in zebrafish embryos.Cardiovasc Res. 2008 Nov 1;80(2):200-8. doi: 10.1093/cvr/cvn177. Epub 2008 Jun 25. Cardiovasc Res. 2008. PMID: 18583338
Cited by
-
The Interaction Between the asb5a and asb5b Subtypes Jointly Regulates the L-R Asymmetrical Development of the Heart in Zebrafish.Int J Mol Sci. 2025 Mar 19;26(6):2765. doi: 10.3390/ijms26062765. Int J Mol Sci. 2025. PMID: 40141403 Free PMC article.
References
-
- Jensen J.H., Conley L.N., Hedegaard J., Nielsen M., Young J.F., Oksbjerg N., Hornshøj H., Bendixen C., Thomsen B. Gene expression profiling of porcine skeletal muscle in the early recovery phase following acute physical activity. Exp. Physiol. 2012;97:833–848. doi: 10.1113/expphysiol.2011.063727. - DOI - PubMed
-
- Zhang S.X., Garcia-Gras E., Wycuff D.R., Marriot S.J., Kadeer N., Yu W., Olson E.N., Garry D.J., Parmacek M.S., Schwartz R.J. Identification of Direct Serum-response Factor Gene Targets during Me2SO-induced P19 Cardiac Cell Differentiation. J. Biol. Chem. 2005;280:19115–19126. doi: 10.1074/jbc.M413793200. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

