SARS-CoV-2 Spike Protein Intensifies Cerebrovascular Complications in Diabetic hACE2 Mice through RAAS and TLR Signaling Activation
- PMID: 38003584
- PMCID: PMC10671133
- DOI: 10.3390/ijms242216394
SARS-CoV-2 Spike Protein Intensifies Cerebrovascular Complications in Diabetic hACE2 Mice through RAAS and TLR Signaling Activation
Abstract
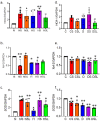
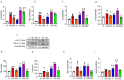
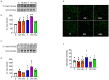
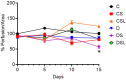
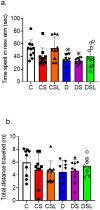
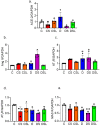
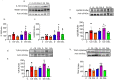
Diabetics are more vulnerable to SARS-CoV-2 neurological manifestations. The molecular mechanisms of SARS-CoV-2-induced cerebrovascular dysfunction in diabetes are unclear. We hypothesize that SARS-CoV-2 exacerbates diabetes-induced cerebrovascular oxidative stress and inflammation via activation of the destructive arm of the renin-angiotensin-aldosterone system (RAAS) and Toll-like receptor (TLR) signaling. SARS-CoV-2 spike protein was injected in humanized ACE2 transgenic knock-in mice. Cognitive functions, cerebral blood flow, cerebrovascular architecture, RAAS, and TLR signaling were used to determine the effect of SARS-CoV-2 spike protein in diabetes. Studies were mirrored in vitro using human brain microvascular endothelial cells treated with high glucose-conditioned media to mimic diabetic conditions. Spike protein exacerbated diabetes-induced cerebrovascular oxidative stress, inflammation, and endothelial cell death resulting in an increase in vascular rarefaction and diminished cerebral blood flow. SARS-CoV-2 spike protein worsened cognitive dysfunction in diabetes compared to control mice. Spike protein enhanced the destructive RAAS arm at the expense of the RAAS protective arm. In parallel, spike protein significantly exacerbated TLR signaling in diabetes, aggravating inflammation and cellular apoptosis vicious circle. Our study illustrated that SAR-CoV-2 spike protein intensified RAAS and TLR signaling in diabetes, increasing cerebrovascular damage and cognitive dysfunction.
Keywords: RAAS; SARS-CoV-2 spike protein; TLR signaling; cerebrovasculature; diabetes; hACE2 KI mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Timing matters in the use of renin-angiotensin system modulators and COVID-related cognitive and cerebrovascular dysfunction.PLoS One. 2024 Jul 29;19(7):e0304135. doi: 10.1371/journal.pone.0304135. eCollection 2024. PLoS One. 2024. PMID: 39074114 Free PMC article.
-
SARS-CoV-2 Spike Protein Exacerbates Thromboembolic Cerebrovascular Complications in Humanized ACE2 Mouse Model.Transl Stroke Res. 2025 Aug;16(4):1214-1228. doi: 10.1007/s12975-024-01301-5. Epub 2024 Oct 2. Transl Stroke Res. 2025. PMID: 39354270 Free PMC article.
-
SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein.Neurobiol Dis. 2021 Dec;161:105561. doi: 10.1016/j.nbd.2021.105561. Epub 2021 Nov 13. Neurobiol Dis. 2021. PMID: 34780863 Free PMC article.
-
SARS-CoV-2, ACE2 expression, and systemic organ invasion.Physiol Genomics. 2021 Feb 1;53(2):51-60. doi: 10.1152/physiolgenomics.00087.2020. Epub 2020 Dec 4. Physiol Genomics. 2021. PMID: 33275540 Free PMC article. Review.
-
Intrinsic disorder perspective of an interplay between the renin-angiotensin-aldosterone system and SARS-CoV-2.Infect Genet Evol. 2020 Nov;85:104510. doi: 10.1016/j.meegid.2020.104510. Epub 2020 Aug 24. Infect Genet Evol. 2020. PMID: 32853823 Free PMC article. Review.
Cited by
-
The Intersection of SARS-CoV-2 and Diabetes.Microorganisms. 2025 Jun 14;13(6):1390. doi: 10.3390/microorganisms13061390. Microorganisms. 2025. PMID: 40572277 Free PMC article. Review.
-
Timing matters in the use of renin-angiotensin system modulators and COVID-related cognitive and cerebrovascular dysfunction.PLoS One. 2024 Jul 29;19(7):e0304135. doi: 10.1371/journal.pone.0304135. eCollection 2024. PLoS One. 2024. PMID: 39074114 Free PMC article.
-
Mechanisms of endothelial activation, hypercoagulation and thrombosis in COVID-19: a link with diabetes mellitus.Cardiovasc Diabetol. 2024 Feb 20;23(1):75. doi: 10.1186/s12933-023-02097-8. Cardiovasc Diabetol. 2024. PMID: 38378550 Free PMC article. Review.
-
SARS-CoV-2 Spike Protein Exacerbates Thromboembolic Cerebrovascular Complications in Humanized ACE2 Mouse Model.Transl Stroke Res. 2025 Aug;16(4):1214-1228. doi: 10.1007/s12975-024-01301-5. Epub 2024 Oct 2. Transl Stroke Res. 2025. PMID: 39354270 Free PMC article.
-
Innate immune sensors and regulators at the blood brain barrier: focus on toll-like receptors and inflammasomes as mediators of neuro-immune crosstalk and inflammation.J Neuroinflammation. 2025 Feb 15;22(1):39. doi: 10.1186/s12974-025-03360-3. J Neuroinflammation. 2025. PMID: 39955600 Free PMC article. Review.
References
-
- CDC. [(accessed on 1 October 2023)];2023 Available online: https://covid.cdc.gov/covid-data-tracker/#cases-deaths-testing-trends.
-
- Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., Yee N.T.S., Liu C., Nerurkar S.N., Kai J.C.Y., et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. - DOI - PMC - PubMed
-
- Premraj L., Kannapadi N.V., Briggs J., Seal S.M., Battaglini D., Fanning J., Suen J., Robba C., Fraser J., Cho S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022;434:120162. doi: 10.1016/j.jns.2022.120162. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

