Alkyl Derivatives of Perylene Photosensitizing Antivirals: Towards Understanding the Influence of Lipophilicity
- PMID: 38003673
- PMCID: PMC10671050
- DOI: 10.3390/ijms242216483
Alkyl Derivatives of Perylene Photosensitizing Antivirals: Towards Understanding the Influence of Lipophilicity
Abstract
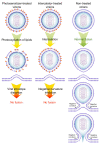
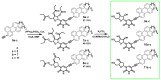
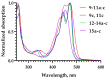
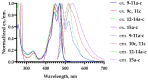
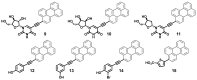
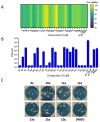
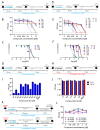
Amphipathic perylene derivatives are broad-spectrum antivirals against enveloped viruses that act as fusion inhibitors in a light-dependent manner. The compounds target the lipid bilayer of the viral envelope using the lipophilic perylene moiety and photogenerating singlet oxygen, thereby causing damage to unsaturated lipids. Previous studies show that variation of the polar part of the molecule is important for antiviral activity. Here, we report modification of the lipophilic part of the molecule, perylene, by the introduction of 4-, 8-, and 12-carbon alkyls into position 9(10) of the perylene residue. Using Friedel-Crafts acylation and Wolff-Kishner reduction, three 3-acetyl-9(10)-alkylperylenes were synthesized from perylene and used to prepare 9 nucleoside and 12 non-nucleoside amphipathic derivatives. These compounds were characterized as fluorophores and singlet oxygen generators, as well as tested as antivirals against herpes virus-1 (HSV-1) and vesicular stomatitis virus (VSV), both known for causing superficial skin/mucosa lesions and thus serving as suitable candidates for photodynamic therapy. The results suggest that derivatives with a short alkyl chain (butyl) have strong antiviral activity, whereas the introduction of longer alkyl substituents (n = 8 and 12) to the perylenyethynyl scaffold results in a dramatic reduction of antiviral activity. This phenomenon is likely attributable to the increased lipophilicity of the compounds and their ability to form insoluble aggregates. Moreover, molecular dynamic studies revealed that alkylated perylene derivatives are predominately located closer to the middle of the bilayer compared to non-alkylated derivatives. The predicted probability of superficial positioning correlated with antiviral activity, suggesting that singlet oxygen generation is achieved in the subsurface layer of the membrane, where the perylene group is more accessible to dissolved oxygen.
Keywords: antivirals; lipophilicity; perylene; photosensitizers; singlet oxygen.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Jurak I., Cokarić Brdovčak M., Djaković L., Bertović I., Knežević K., Lončarić M., Jurak Begonja A., Malatesti N. Photodynamic inhibition of herpes simplex virus 1 infection by tricationic amphiphilic porphyrin with a long alkyl chain. Pharmaceutics. 2023;15:956. doi: 10.3390/pharmaceutics15030956. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

