Emerging Role of Circular RNAs in Hepatocellular Carcinoma Immunotherapy
- PMID: 38003674
- PMCID: PMC10671287
- DOI: 10.3390/ijms242216484
Emerging Role of Circular RNAs in Hepatocellular Carcinoma Immunotherapy
Abstract
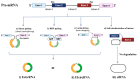
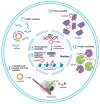
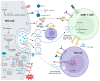
Hepatocellular carcinoma (HCC) is a highly fatal malignancy with limited therapeutic options and high recurrence rates. Recently, immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) have emerged as a new paradigm shift in oncology. ICIs, such as programmed cell death protein 1 (PD-1) inhibitors, have provided a new source of hope for patients with advanced HCC. Yet, the eligibility criteria of HCC patients for ICIs are still a missing piece in the puzzle. Circular RNAs (circRNAs) have recently emerged as a new class of non-coding RNAs that play a fundamental role in cancer pathogenesis. Structurally, circRNAs are resistant to exonucleolytic degradation and have a longer half-life than their linear counterparts. Functionally, circRNAs possess the capability to influence various facets of the tumor microenvironment, especially at the HCC tumor-immune synapse. Notably, circRNAs have been observed to control the expression of immune checkpoint molecules within tumor cells, potentially impeding the therapeutic effectiveness of ICIs. Therefore, this renders them potential cancer-immune biomarkers for diagnosis, prognosis, and therapeutic regimen determinants. In this review, the authors shed light on the structure and functional roles of circRNAs and, most importantly, highlight the promising roles of circRNAs in HCC immunomodulation and their potential as promising biomarkers and immunotherapeutic regimen determinants.
Keywords: circular RNAs (circRNAs); cytotoxic T lymphocytes; hepatocellular carcinoma (HCC); immunotherapy; natural killer cells; tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

