Heterotypic Stressors Unmask Behavioral Influences of PMAT Deficiency in Mice
- PMID: 38003684
- PMCID: PMC10671398
- DOI: 10.3390/ijms242216494
Heterotypic Stressors Unmask Behavioral Influences of PMAT Deficiency in Mice
Abstract
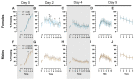
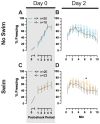
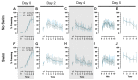
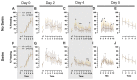
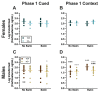
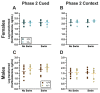
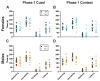
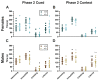
Certain life stressors having enduring physiological and behavioral consequences, in part by eliciting dramatic signaling shifts in monoamine neurotransmitters. High monoamine levels can overwhelm selective transporters like the serotonin transporter. This is when polyspecific transporters like plasma membrane monoamine transporter (PMAT, Slc29a4) are hypothesized to contribute most to monoaminergic signaling regulation. Here, we employed two distinct counterbalanced stressors-fear conditioning and swim stress-in mice to systematically determine how reductions in PMAT function affect heterotypic stressor responsivity. We hypothesized that male heterozygotes would exhibit augmented stressor responses relative to female heterozygotes. Decreased PMAT function enhanced context fear expression, an effect unexpectedly obscured by a sham stress condition. Impaired cued fear extinction retention and enhanced context fear expression in males were conversely unmasked by a sham swim condition. Abrogated corticosterone levels in male heterozygotes that underwent swim stress after context fear conditioning did not map onto any measured behaviors. In sum, male heterozygous mouse fear behaviors proved malleable in response to preceding stressor or sham stress exposure. Combined, these data indicate that reduced male PMAT function elicits a form of stress-responsive plasticity. Future studies should assess how PMAT is differentially affected across sexes and identify downstream consequences of the stress-shifted corticosterone dynamics.
Keywords: behavior; corticosterone; fear conditioning; mice; sex differences; stress; swim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Update of
-
Heterotypic stressors unmask behavioral influences of PMAT deficiency in mice.bioRxiv [Preprint]. 2023 Nov 13:2023.08.30.555632. doi: 10.1101/2023.08.30.555632. bioRxiv. 2023. Update in: Int J Mol Sci. 2023 Nov 18;24(22):16494. doi: 10.3390/ijms242216494. PMID: 37693400 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

