Interaction of TSG101 with the PTAP Motif in Distinct Locations of Gag Determines the Incorporation of HTLV-1 Env into the Retroviral Virion
- PMID: 38003710
- PMCID: PMC10671467
- DOI: 10.3390/ijms242216520
Interaction of TSG101 with the PTAP Motif in Distinct Locations of Gag Determines the Incorporation of HTLV-1 Env into the Retroviral Virion
Abstract
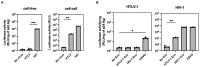
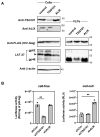
Human T-cell tropic virus type 1 (HTLV-1) is known to be mainly transmitted by cell-to-cell contact due to the lower infectivity of the cell-free virion. However, the reasons why cell-free HTLV-1 infection is poor remain unknown. In this study, we found that the retrovirus pseudotyped with HTLV-1 viral envelope glycoprotein (Env) was infectious when human immunodeficiency virus type 1 (HIV-1) was used to produce the virus. We found that the incorporation of HTLV-1 Env into virus-like particles (VLPs) was low when HTLV-1 Gag was used to produce VLPs, whereas VLPs produced using HIV-1 Gag efficiently incorporated HTLV-1 Env. The production of VLPs using Gag chimeras between HTLV-1 and HIV-1 Gag and deletion mutants of HIV-1 Gag showed that the p6 domain of HIV-1 Gag was responsible for the efficient incorporation of HTLV-1 Env into the VLPs. Further mutagenic analyses of the p6 domain of HIV-1 Gag revealed that the PTAP motif in the p6 domain of HIV-1 Gag facilitates the incorporation of HTLV-1 Env into VLPs. Since the PTAP motif is known to interact with tumor susceptibility gene 101 (TSG101) during the budding process, we evaluated the effect of TSG101 knockdown on the incorporation of HTLV-1 Env into VLPs. We found that TSG101 knockdown suppressed the incorporation of HTLV-1 Env into VLPs and decreased the infectivity of cell-free HIV-1 pseudotyped with HTLV-1 Env. Our results suggest that the interaction of TSG101 with the PTAP motif of the retroviral L domain is involved not only in the budding process but also in the efficient incorporation of HTLV-1 Env into the cell-free virus.
Keywords: HTLV-1; PTAP; TSG101; infectivity; retroviral Gag.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Role of the human T-cell leukemia virus type 1 PTAP motif in Gag targeting and particle release.J Virol. 2006 Apr;80(7):3634-43. doi: 10.1128/JVI.80.7.3634-3643.2006. J Virol. 2006. PMID: 16537631 Free PMC article.
-
Late assembly motifs of human T-cell leukemia virus type 1 and their relative roles in particle release.J Virol. 2004 Jun;78(12):6636-48. doi: 10.1128/JVI.78.12.6636-6648.2004. J Virol. 2004. PMID: 15163754 Free PMC article.
-
Nedd4.1-mediated ubiquitination and subsequent recruitment of Tsg101 ensure HTLV-1 Gag trafficking towards the multivesicular body pathway prior to virus budding.J Cell Sci. 2004 May 1;117(Pt 11):2357-67. doi: 10.1242/jcs.01095. J Cell Sci. 2004. PMID: 15126635
-
New insights into HTLV-1 particle structure, assembly, and Gag-Gag interactions in living cells.Viruses. 2011 Jun;3(6):770-93. doi: 10.3390/v3060770. Epub 2011 Jun 14. Viruses. 2011. PMID: 21994753 Free PMC article. Review.
-
Mechanisms for Env glycoprotein acquisition by retroviruses.AIDS Res Hum Retroviruses. 2011 Mar;27(3):239-47. doi: 10.1089/AID.2010.0350. Epub 2011 Feb 22. AIDS Res Hum Retroviruses. 2011. PMID: 21247353 Free PMC article. Review.
Cited by
-
M-Sec promotes the accumulation of intracellular HTLV-1 Gag puncta and the incorporation of Env into viral particles.PLoS Pathog. 2025 Jan 27;21(1):e1012919. doi: 10.1371/journal.ppat.1012919. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39869648 Free PMC article.
References
-
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K.I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

