Leucine-Rich Alpha-2 Glycoprotein 1 Accumulates in Complicated Atherosclerosis and Promotes Calcification
- PMID: 38003727
- PMCID: PMC10671851
- DOI: 10.3390/ijms242216537
Leucine-Rich Alpha-2 Glycoprotein 1 Accumulates in Complicated Atherosclerosis and Promotes Calcification
Abstract
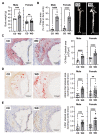
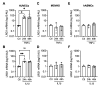
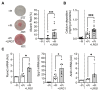
Atherosclerosis is the primary cause of cardiovascular disease. The development of plaque complications, such as calcification and neo-angiogenesis, strongly impacts plaque stability and is a good predictor of mortality in patients with atherosclerosis. Despite well-known risk factors of plaque complications, such as diabetes mellitus and chronic kidney disease, the mechanisms involved are not fully understood. We and others have identified that the concentration of circulating leucine-rich α-2 glycoprotein 1 (LRG1) was increased in diabetic and chronic kidney disease patients. Using apolipoprotein E knockout mice (ApoE-/-) (fed with Western diet) that developed advanced atherosclerosis and using human carotid endarterectomy, we showed that LRG1 accumulated into an atherosclerotic plaque, preferentially in calcified areas. We then investigated the possible origin of LRG1 and its functions on vascular cells and found that LRG1 expression was specifically enhanced in endothelial cells via inflammatory mediators and not in vascular smooth muscle cells (VSMC). Moreover, we identified that LRG1 was able to induce calcification and SMAD1/5-signaling pathways in VSMC. In conclusion, our results identified for the first time that LRG1 is a direct contributor to vascular calcification and suggest a role of this molecule in the development of plaque complications in patients with atherosclerosis.
Keywords: LRG1; atherosclerosis; calcification; vascular smooth muscle cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Van Rosendael A.R., Narula J., Lin F.Y., van den Hoogen I.J., Gianni U., Al Hussein Alawamlh O., Dunham P.C., Peña J.M., Lee S.-E., Andreini D., et al. Association of High-Density Calcified 1K Plaque with Risk of Acute Coronary Syndrome. JAMA Cardiol. 2020;5:282–290. doi: 10.1001/jamacardio.2019.5315. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

