Establishment of an Animal Model Scheme of Strongyloides stercoralis-Infected Meriones meridianus
- PMID: 38003750
- PMCID: PMC10675186
- DOI: 10.3390/pathogens12111285
Establishment of an Animal Model Scheme of Strongyloides stercoralis-Infected Meriones meridianus
Abstract
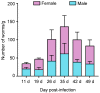
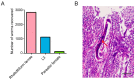
Studying parasitic nematodes, which generate a massive hazard to animal health, is more difficult than studying free-living nematodes as appropriate animal models are essential, and the relationship between parasites and hosts is extremely complex. Strongyloides stercoralis is an intestinal nematode parasite that mainly infects dogs, humans and other primates. Currently, S. stercoralis worms needed for research mainly rely on their natural host, the dog. This study explored a method of using Meriones meridianus as a model for S. stercoralis. The immunosuppressed M. meridianus were infected with S. stercoralis subcutaneously, and post-parasitic, first-stage larvae (PP L1) were detected in the faeces, with more larvae in female gerbils. In addition, parasitic females (PFs), third-stage larvae (L3s) and rhabditiform larvae were found primarily in the small intestines and lungs of infected gerbils. The PFs and auto-infective third-stage larvae (aL3s) obtained from M. meridianus are morphologically identical to those obtained from beagles and Meriones unguiculatus. Moreover, the infection of S. stercoralis caused changes to biochemical indicators in the serum and in the physiology of M. meridianus. The results demonstrated that M. meridianus can be infected by S. stercoralis, and this model provides a great tool for exploring the biological processes of this parasite and its interaction with the host.
Keywords: Meriones meridianus; Strongyloides stercoralis; animal model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The Experimental Infections of the Human Isolate of Strongyloides Stercoralis in a Rodent Model (The Mongolian Gerbil, Meriones Unguiculatus).Pathogens. 2019 Feb 5;8(1):21. doi: 10.3390/pathogens8010021. Pathogens. 2019. PMID: 30764580 Free PMC article.
-
Strongyloides stercoralis: high worm population density leads to autoinfection in the jird (Meriones unguiculatus).Exp Parasitol. 2002 Mar;100(3):173-8. doi: 10.1016/s0014-4894(02)00014-0. Exp Parasitol. 2002. PMID: 12173402
-
Strongyloides stercoralis: hyperinfection in immunosuppressed dogs.Exp Parasitol. 1984 Jun;57(3):287-96. doi: 10.1016/0014-4894(84)90103-6. Exp Parasitol. 1984. PMID: 6723900
-
Strongyloides infection in rodents: immune response and immune regulation.Parasitology. 2017 Mar;144(3):295-315. doi: 10.1017/S0031182016000111. Epub 2016 Feb 24. Parasitology. 2017. PMID: 26905057 Review.
-
Chemo- and thermosensory neurons: structure and function in animal parasitic nematodes.Vet Parasitol. 1999 Aug 1;84(3-4):297-316. doi: 10.1016/s0304-4017(99)00037-0. Vet Parasitol. 1999. PMID: 10456420 Review.
Cited by
-
The efficacy and safety of efgartigimod for refractory myasthenia gravis: a systematic review and meta-analysis.Eur J Med Res. 2025 Aug 20;30(1):775. doi: 10.1186/s40001-025-03057-6. Eur J Med Res. 2025. PMID: 40830529 Free PMC article.
References
-
- Alvarado-Gonzalez J.C., Alvis-Zakzuk N.R., Castillo-Saavedra D.E., Lozada-Martinez I.D., Picon-Jaimes Y.A., Narvaez-Rojas A.R., Zakzuk J. Impact of helminthiasis on gestational anemia in low- and middle-income countries: A systematic review and meta-analysis of more than 19,000 women. Le. Infezioni. In. Medicina. 2022;31:36–48. - PMC - PubMed
-
- Charlier J., Rinaldi L., Musella V., Ploeger H.W., Chartier C., Vineer H.R., Hinney B., von Samson-Himmelstjerna G., Bacescu B., Mickiewicz M., et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020;182:105103. doi: 10.1016/j.prevetmed.2020.105103. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

