Influence of Maternal BLV Infection on miRNA and tRF Expression in Calves
- PMID: 38003777
- PMCID: PMC10674961
- DOI: 10.3390/pathogens12111312
Influence of Maternal BLV Infection on miRNA and tRF Expression in Calves
Abstract
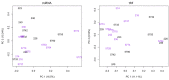
Small non-coding RNAs, such as microRNAs (miRNA) and tRNA-derived fragments (tRF), are known to be involved in post-transcriptional gene regulation. Research has provided evidence that small RNAs may influence immune development in calves. Bovine leukosis is a disease in cattle caused by Bovine Leukemia Virus (BLV) that leads to increased susceptibility to opportunistic pathogens. No research has addressed the potential influence that a maternal BLV infection may have on gene regulation through the differential expression of miRNAs or tRFs in progeny. Blood samples from 14-day old Holstein calves born to BLV-infected dams were collected. Antibodies for BLV were assessed using ELISA and levels of BLV provirus were assessed using qPCR. Total RNA was extracted from whole blood samples for small RNA sequencing. Five miRNAs (bta-miR-1, bta-miR-206, bta-miR-133a, bta-miR-133b, and bta-miR-2450d) and five tRFs (tRF-36-8JZ8RN58X2NF79E, tRF-20-0PF05B2I, tRF-27-W4R951KHZKK, tRF-22-S3M8309NF, and tRF-26-M87SFR2W9J0) were dysregulated in calves born to BLV-infected dams. The miRNAs appear to be involved in the gene regulation of immunological responses and muscle development. The tRF subtypes and parental tRNA profiles in calves born to infected dams appear to be consistent with previous publications in adult cattle with BLV infection. These findings offer insight into how maternal BLV infection status may impact the development of offspring.
Keywords: BLV; bovine leukemia virus; cattle; microRNAs; tRNA-derived fragments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Exploration of microRNA Biomarkers in Blood Small Extracellular Vesicles for Enzootic Bovine Leukosis.Microorganisms. 2023 Aug 28;11(9):2173. doi: 10.3390/microorganisms11092173. Microorganisms. 2023. PMID: 37764017 Free PMC article.
-
Characterization of microRNA expression in B cells derived from Japanese black cattle naturally infected with bovine leukemia virus by deep sequencing.PLoS One. 2021 Sep 10;16(9):e0256588. doi: 10.1371/journal.pone.0256588. eCollection 2021. PLoS One. 2021. PMID: 34506539 Free PMC article.
-
Characterization of miRNAs in Milk Small Extracellular Vesicles from Enzootic Bovine Leukosis Cattle.Int J Mol Sci. 2022 Sep 15;23(18):10782. doi: 10.3390/ijms231810782. Int J Mol Sci. 2022. PMID: 36142686 Free PMC article.
-
Bovine Leukemia Virus Infection in Neonatal Calves. Risk Factors and Control Measures.Front Vet Sci. 2018 Oct 25;5:267. doi: 10.3389/fvets.2018.00267. eCollection 2018. Front Vet Sci. 2018. PMID: 30410920 Free PMC article. Review.
-
Detrimental effect of bovine leukemia virus (BLV) on the immunological state of cattle.Vet Immunol Immunopathol. 1996 Nov;54(1-4):293-302. doi: 10.1016/s0165-2427(96)05706-6. Vet Immunol Immunopathol. 1996. PMID: 8988875 Review.
Cited by
-
tRNA-derived small RNAs in disease immunity.Theranostics. 2025 Jan 1;15(1):245-257. doi: 10.7150/thno.102650. eCollection 2025. Theranostics. 2025. PMID: 39744232 Free PMC article. Review.
References
-
- Arsenault D., St-Amour I., Cisbani G., Rousseau L.S., Cicchetti F. The different effects of LPS and poly I:C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain Behav. Immun. 2014;38:77–90. doi: 10.1016/j.bbi.2013.12.016. - DOI - PubMed
LinkOut - more resources
Full Text Sources

