Pathomorphological Findings and Infectious Diseases in Selected European Brown Hare (Lepus europaeus Pallas, 1778) Populations from Schleswig-Holstein, Germany
- PMID: 38003782
- PMCID: PMC10675426
- DOI: 10.3390/pathogens12111317
Pathomorphological Findings and Infectious Diseases in Selected European Brown Hare (Lepus europaeus Pallas, 1778) Populations from Schleswig-Holstein, Germany
Abstract
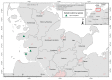
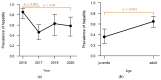
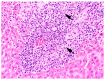
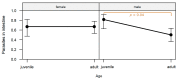
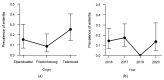
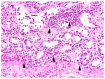
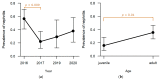
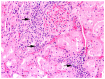
In the northernmost German federal state Schleswig-Holstein, populations of European brown hares (Lepus europaeus Pallas, 1778) show diverse densities and varying courses over the years. To examine differences in pathomorphological findings and infectious diseases as possible reasons for varying population dynamics, we assessed 155 hunted hares from three locations in Schleswig-Holstein from 2016 to 2020. We investigated the association of location, year, age, and sex of animals to certain pathomorphological findings and infectious diseases. Frequent pathomorphological findings were intestinal parasites (63.9%), hepatitis (55.5%), nephritis (31.0%), steatitis (23.2%), enteritis (13.5%), and pneumonia (5.2%). Body condition differed significantly between locations, and the prevalence of pneumonia was significantly higher in females. Enteritis was not detected in 2019, when much more juveniles were sampled. Hepatitis and nephritis occurred significantly more often in 2016 and among adults. Additionally, more adults showed hepatitis with concurrent serotitre for European brown hare syndrome virus (EBHSV), while intestinal parasitosis as well as high excretion rates of coccidia were more common in juveniles. Sampled animals showed high infection rates with Eimeria spp. (96.1%), Trichostrongylus spp. (52.0%), Graphidium strigosum (41.2%), and a high seroprevalence (90.9%) for EBHSV, without severe symptoms. This study revealed a low prevalence of infectious pathogens, but a high prevalence of chronic inflammations of unknown origin in the tested brown hare populations. Overall, our results indicate a rather minor importance of infectious diseases for observed population dynamics of analysed hare populations in Schleswig-Holstein.
Keywords: EBHSV; Eimeria spp.; Graphidium strigosum; Lepus europaeus; Trichostrongylus spp.; hepatitis; nephritis; steatitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Status of Infectious Diseases in Free-Ranging European Brown Hares (Lepus europaeus) Found Dead between 2017 and 2020 in Schleswig-Holstein, Germany.Pathogens. 2023 Feb 2;12(2):239. doi: 10.3390/pathogens12020239. Pathogens. 2023. PMID: 36839511 Free PMC article.
-
Epizootiologic and ecologic investigations of European brown hares (Lepus europaeus) in selected populations from Schleswig-Holstein, Germany.J Wildl Dis. 2003 Oct;39(4):751-61. doi: 10.7589/0090-3558-39.4.751. J Wildl Dis. 2003. PMID: 14733269
-
Endoparasites of the European Hare (Lepus Europaeus) (Pallas, 1778) in Central Italy.Helminthologia. 2018 Jun 1;55(2):127-133. doi: 10.2478/helm-2018-0011. eCollection 2018 Jun. Helminthologia. 2018. PMID: 31662638 Free PMC article.
-
European Brown hare (Lepus europaeus) as a source of emerging and re-emerging pathogens of Public Health importance: A review.Vet Med Sci. 2020 Aug;6(3):550-564. doi: 10.1002/vms3.248. Epub 2020 Feb 23. Vet Med Sci. 2020. PMID: 32088933 Free PMC article. Review.
-
Epidemiology and diagnosis of the European brown hare syndrome in Scandinavian countries: a review.Rev Sci Tech. 1991 Jun;10(2):453-8. doi: 10.20506/rst.10.2.555. Rev Sci Tech. 1991. PMID: 1760585 Review.
References
-
- Hackländer K., Schai-Braun S. Lepus europaeus Pallas, 1778 European hare. In: Smith A.T., Johnston C.H., Alves P.C., Hackländer K., editors. Lagomorphs Pikas, Rabbits, and Hares of the World. Johns Hopkins University Press; Baltimore, MD, USA: 2018. pp. 187–190.
-
- Alves P.C., Hackländer K. Lagomorph species: Geographical distribution and conservation status. In: Alves P.C., Ferrand N., Hackländer K., editors. Lagomorph Biology: Evolution, Ecology, and Conservation. Springer; Berlin/Heidelberg, Germany: 2008. pp. 395–406.
-
- Wincentz T.-L. Ph.D. Thesis. Aarhus University; Aarhus, Denmark: 2009. Identifying Causes for Population Decline of Brown Hare (Lepus europaeus) in Agricultural Landscapes in Denmark.
-
- Hutchings M.R., Harris S. The Current Status of the Brown Hare (Lepus europaeus) in Britain. Joint Nature Conservation Committee; Bristol, UK: 1996.
LinkOut - more resources
Full Text Sources
Miscellaneous

