Immune Portrayal of a New Therapy Targeting Microbiota in an Animal Model of Psoriasis
- PMID: 38003872
- PMCID: PMC10672519
- DOI: 10.3390/jpm13111556
Immune Portrayal of a New Therapy Targeting Microbiota in an Animal Model of Psoriasis
Abstract
Background: Despite all the available treatments, psoriasis remains incurable; therefore, finding personalized therapies is a continuous challenge. Psoriasis is linked to a gut microbiota imbalance, highlighting the importance of the gut-skin axis and its inflammatory mediators. Restoring this imbalance can open new perspectives in psoriasis therapy. We investigated the effect of purified IgY raised against pathological human bacteria antibiotic-resistant in induced murine psoriatic dermatitis (PSO).
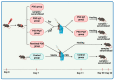
Methods: To evaluate the immune portrayal in an imiquimod experimental model, before and after IgY treatment, xMAP array and flow cytometry were used.
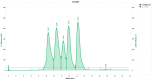
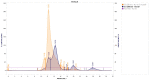
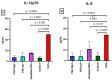
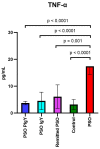
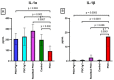
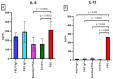
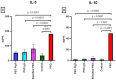
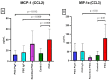
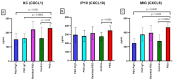
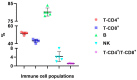
Results: There were significant changes in IL-1α,β, IL-5, IL-6, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17a, IFN-γ, TNF-α, IP-10/CXCL10, MCP-1/CCL2, MIP-1α/CCL3, MIP-1β/CCL4, MIG/CXCL9, and KC/CXCL1 serum levels. T (CD3ε+), B (CD19+) and NK (NK1.1+) cells were also quantified. In our model, TNF-α, IL-6, and IL-1β cytokines and CXCL1 chemokine have extremely high circulatory levels in the PSO group. Upon experimental therapy, the cytokine serum values were not different between IgY-treated groups and spontaneously remitted PSO.
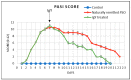
Conclusions: Using the murine model of psoriatic dermatitis, we show that the orally purified IgY treatment can lead to an improvement in skin lesion healing along with the normalization of cellular and humoral immune parameters.
Keywords: IgY; cytokines; gut–skin axis; inflammation; psoriatic dermatitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cytokine profile characterization of naïve patients with psoriasis and psoriatic arthritis: implications for a pathogenic disease continuum.Front Immunol. 2023 Jul 13;14:1229516. doi: 10.3389/fimmu.2023.1229516. eCollection 2023. Front Immunol. 2023. PMID: 37520537 Free PMC article.
-
Unconventional Therapy with IgY in a Psoriatic Mouse Model Targeting Gut Microbiome.J Pers Med. 2021 Aug 26;11(9):841. doi: 10.3390/jpm11090841. J Pers Med. 2021. PMID: 34575618 Free PMC article.
-
Chemokine profile in women with moderate to severe anxiety and depression during pregnancy.BMC Pregnancy Childbirth. 2021 Dec 4;21(1):807. doi: 10.1186/s12884-021-04225-2. BMC Pregnancy Childbirth. 2021. PMID: 34863117 Free PMC article.
-
Disruption of CCR5 signaling to treat COVID-19-associated cytokine storm: Case series of four critically ill patients treated with leronlimab.J Transl Autoimmun. 2021;4:100083. doi: 10.1016/j.jtauto.2021.100083. Epub 2021 Jan 6. J Transl Autoimmun. 2021. PMID: 33521616 Free PMC article. Review.
-
Microbiota and IL-33/31 Axis Linkage: Implications and Therapeutic Perspectives in Atopic Dermatitis and Psoriasis.Biomolecules. 2023 Jul 10;13(7):1100. doi: 10.3390/biom13071100. Biomolecules. 2023. PMID: 37509136 Free PMC article. Review.
Cited by
-
Long-Term Oral Administration of Hyperimmune Egg-Based IgY-Rich Formulations Induces Mucosal Immune Response and Systemic Increases of Cytokines Involved in Th2- and Th17-Type Immune Responses in C57BL/6 Mice.Int J Mol Sci. 2024 Aug 9;25(16):8701. doi: 10.3390/ijms25168701. Int J Mol Sci. 2024. PMID: 39201385 Free PMC article.
References
-
- Ion A., Dorobanțu A.M., Popa L.G., Mihai M.M., Orzan O.A. Risks of Biologic Therapy and the Importance of Multidisciplinary Approach for an Accurate Management of Patients with Moderate-Severe Psoriasis and Concomitant Diseases. Biology. 2022;11:808. doi: 10.3390/biology11060808. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

