Analysis of New Colposcopy Techniques in the Diagnosis and Evolution of SIL/CIN: Comparison of Colposcopy with the DSI System (COLPO-DSI Study)
- PMID: 38003920
- PMCID: PMC10672663
- DOI: 10.3390/jpm13111605
Analysis of New Colposcopy Techniques in the Diagnosis and Evolution of SIL/CIN: Comparison of Colposcopy with the DSI System (COLPO-DSI Study)
Abstract
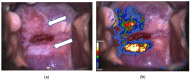
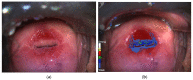
Compared with conventional colposcopy, colposcopy assisted by DSI-map increases the detection of HSIL/CIN2+ and might help to identify the lesions more likely to regress.
Introduction: Comparison of the performance of colposcopy assisted by dynamic spectral imaging (C-DSI) with that of conventional colposcopy (CC) in the diagnosis of cervical intraepithelial neoplasia (HSIL/CIN2 or CIN3).
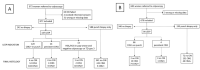
Materials and methods: A total of 1655 women were referred for colposcopy between 2012 and 2020 and included in the study. Of that total, 973 were examined by the same colposcopist with C-DSI, and 682 with CC. Comparisons between CC and C-DSI were made by using the histological diagnosis performed with a punch biopsy or loop electrosurgical excision procedure (LEEP) as the gold standard. A follow-up study was conducted until 2021 to detect progression to HSIL/CIN2 at 6, 12 and 24 months after first examination.
Results: C-DSI provided higher sensitivity for the diagnosis of HSIL/CIN2 or CIN 3 than CC (sensitivity of 76.8% and 86.6% vs. 54.2% and 72.2%, respectively). In negative or ASCUS/LSIL Pap smear results, C-DSI showed higher sensitivity than CC (sensitivity of 66.7% and 61.5% vs. 21.4% and 33.3%, respectively). In contrast, these differences were not observed in high-grade Pap smears. The sensitivity of C-DSI in cases with HPV16/18 infection was stronger than that of CC (73.53% vs. 56.67%). The sensitivity of C-DSI to detect the progression to HSIL/CIN2+ during follow-up was 30, 17.6 and 35.7% at 6, 12 and 24 months, respectively.
Conclusions: The present study shows that C-DSI in women referred for colposcopy increases the HSIL/CIN 2-3 detection rate compared to conventional colposcopy. Nevertheless, C-DSI does not seem to be an important tool to predict the evolution of the lesions during follow-up.
Keywords: cervical intraepithelial neoplasia; colposcopy; dynamic spectral imaging; dysis; hpv; human papillomavirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Value of colposcopy with dynamic spectral imaging in the detection and evolution of high-grade cervical lesions.Expert Rev Med Devices. 2022 Jun;19(6):525-532. doi: 10.1080/17434440.2022.2104638. Epub 2022 Jul 26. Expert Rev Med Devices. 2022. PMID: 35858486
-
Colposcopy combined with dynamic spectral imaging. A prospective clinical study.Eur J Obstet Gynecol Reprod Biol. 2016 Jan;196:11-6. doi: 10.1016/j.ejogrb.2015.09.007. Epub 2015 Oct 20. Eur J Obstet Gynecol Reprod Biol. 2016. PMID: 26651076
-
Can the dynamic spectral imaging (DSI) color map improve colposcopy examination for precancerous cervical lesions? A prospective evaluation of the DSI color map in a multi-biopsy clinical setting.BMC Womens Health. 2021 Jan 12;21(1):21. doi: 10.1186/s12905-020-01169-1. BMC Womens Health. 2021. PMID: 33435974 Free PMC article.
-
Agreement between colposcopic impression and histological diagnosis among human papillomavirus type 16-positive women: a clinical trial using dynamic spectral imaging colposcopy.BJOG. 2012 Apr;119(5):537-44. doi: 10.1111/j.1471-0528.2012.03280.x. Epub 2012 Feb 3. BJOG. 2012. PMID: 22304443 Clinical Trial.
-
Diagnostic accuracy of colposcopy with dynamic spectral imaging for cytology-negative/high-risk HPV positive (failed test of cure) after large loop excision of the transformation zone (LLETZ) of the cervix: Results of the DySIS colposcopy 1 study.Medicine (Baltimore). 2018 Jan;97(1):e9560. doi: 10.1097/MD.0000000000009560. Medicine (Baltimore). 2018. PMID: 29505536 Free PMC article.
Cited by
-
Predictive value of vaginal lactic acid bacteria changes on occurrence of HR-HPV-infected cervical intraepithelial neoplasia and construction and validation of nomogram model.BMC Cancer. 2025 Jul 24;25(1):1212. doi: 10.1186/s12885-025-14604-z. BMC Cancer. 2025. PMID: 40707911 Free PMC article.
References
-
- International Agency for Research on Cancer. World Health Organization [(accessed on 24 March 2021)]. Available online: https://gco.iarc.fr/today/home.
-
- Arbyn M., Ronco G., Anttila A., Meijer C.J., Poljak M., Ogilvie G., Koliopoulos G., Naucler P., Sankaranarayanan R., Peto J. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30((Suppl. S5)):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. - DOI - PubMed
-
- Segnan N., Anttila A., Karsa L., Ronco G., Törnberg S., Patnick J., De Vuyst H., Dillner J., Franceschi S., Arbyn M., et al., editors. European Guidelines for Quality Assurance in Cervical Cancer Screening. 2nd ed. Publications Office of the European Union; Luxembourg: 2015. - DOI
-
- Torné A., Andía D., Castro M., de la Fuente J., Hernández J.J., López J.A., Martínez J.C., Medina N., Quílez J.C., Ramírez Mena M., et al. AEPCC-Guideline: Colposcopy Guidelines. Standards of Quality. AEPCC Publications; Valencia, Spain: 2018. pp. 1–80.
-
- WHO . Control Integral del Cáncer Cervicouterino: Guía de Prácticas Esenciales. 2nd ed. WHO; Washington, DC, USA: 2016.
LinkOut - more resources
Full Text Sources
Research Materials

