Cemiplimab as First Line Therapy in Advanced Penile Squamous Cell Carcinoma: A Real-World Experience
- PMID: 38003938
- PMCID: PMC10672594
- DOI: 10.3390/jpm13111623
Cemiplimab as First Line Therapy in Advanced Penile Squamous Cell Carcinoma: A Real-World Experience
Abstract
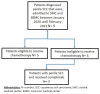
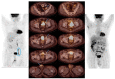
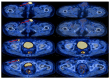
In the treatment of cancer, immune checkpoint inhibitors (ICIs) have demonstrated significantly greater effectiveness compared to conventional cytotoxic or platinum-based chemotherapies. To assess the efficacy of ICI's in penile squamous cell carcinoma (pSCC) we performed a retrospective observational study. We reviewed electronic medical records of patients with penile squamous cell carcinoma (SCC), diagnosed between January 2020 and February 2023. Nine patients were screened, of whom three were ineligible for chemotherapy and received immunotherapy, cemiplimab, in a first-line setting. Each of the three immunotherapy-treated patients achieved almost a complete response (CR) after only a few cycles of therapy. The first patient had cerebral arteritis during treatment and received a high-dose steroid treatment with resolution of the symptoms of arteritis. After tapering down the steroids dose, the patient continued cemiplimab without further toxicity. The other two patients did not have any toxic side effects of the treatment. To the best of our knowledge, this is the first real world report of near CR with cemiplimab as a first-line treatment in penile SCC.
Keywords: cemiplimab; chemotherapy-ineligible; immune checkpoint inhibitors (ICIs); penile carcinoma; squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Douglawi A., Masterson T.M. Opinion in Urology, and Undefined 2019: Penile Cancer Epidemiology and Risk Factors: A Contemporary Review. [(accessed on 7 September 2022)]. Available online: https://journals.lww.com/co-urology/Fulltext/2019/03000/Penile_cancer_ep.... - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

